Neurologic Complications of Peripheral Nerve Blocks
Author: Jeff Gadsden
|
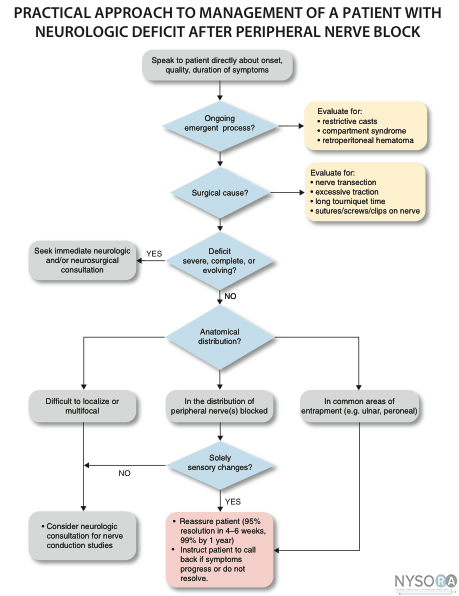
Nerve injury following peripheral nerve blockade (PNB) is a potentially devastating complication that can result in permanent disability. (1) Data from a recent review of published studies suggest that the incidence of neurologic symptoms following PNB varies depending on the anatomic location, ranging from 0.03% for supraclavicular blocks to 0.3% for femoral blocks to up to 3% for interscalene blocks. (2) Fortunately, the vast majority of these neuropathies appear to be temporary rather than permanent neuropathy and resolve over weeks to months. The exact etiology of neurologic injury related to PNB remains unclear in many instances. Suggested etiologies include mechanical trauma from the needle, nerve edema and/or hematoma, pressure effects of the local anesthetic injectate, and neurotoxicity of the injected solutions (both local anesthetics and adjuvants, e.g., epinephrine). (3) Confounding factors that may play a role in nerve injury include preexisting neuropathies (e.g., diabetes mellitus), surgical manipulation, prolonged tourniquet pressure, or compression from postoperative casting. (4) It is well-established that direct injection into peripheral nerves (i.e., accidentally during intramuscular administration) can result in nerve injury. (5) This is one of the reasons why intraneural injections are avoided during peripheral nerve blockade. More recent data however, suggest that intraneural injections are not always associated with nerve injury. This chapter summarizes the clinically relevant considerations regarding the etiology of nerve injury during peripheral nerve blockade. Histology and Histopathology of Peripheral Nerves 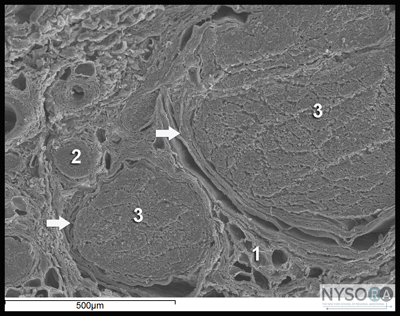 Figure 1: Anatomy of the peripheral nerve as seen on an electron microscopy image. 1-Epineurium, 2-Fascicle, 3-Fascicular bundles (several fascicles bound together), Arrows-perineurium. Knowledge of the functional histology of nerves is essential to understanding the consequences of intraneural injection. Nerves are made up of fascicles supported and enveloped by perineurium and a loose collection of collagen fibers termed the epineurium. The epineurium is easy permeable and carries the nutritive vessels of larger nerves. Each fascicle is made up of bundles of nerve fibers (axons) and their associated Schwann cells held together by a tough squamous epithelial sheath called the perineurium, which acts as a semipermeable barrier to local anesthetics. The nerve fibers are supported within the perineurium by a delicate connective tissue matrix called the endoneurium, which contains capillaries that arise from the larger epineurial vessels. Figure 1 features normal anatomy of a mixed peripheral nerve and relationship of the epineurium and perineurium. Peripheral nerve lesions can be classified in terms of their degree of functional disruption. (6) Neurapraxia refers to a mild insult in which the axons and connective tissue structures supporting them remain intact. This type of injury is often associated with focal demyelination and generally reversible over the course of weeks to several months. Axonal interruption with conservation of the neural connective tissues is termed axonotmesis. Regeneration at a rate of 1 to 2 mm/day occurs, and recovery is generally favorable although not always complete. Neurotmesis represents complete fascicular interruption, including the axons and the connective tissue supporting tissues. Because the nerve is severed, recovery depends on surgical reapproximation of the two stumps. Even with prompt surgical intervention, recovery is often poor. It is important to note that most nerve injuries are mixed, with different fascicles exhibiting characteristics of these three different injury types. The Problem, or is It? Selander et al provided evidence of the deleterious effects of intraneural injection over 30 years ago (7) Indeed, the objective during peripheral nerve blockade has been to deposit local anesthetic in the vicinity, but not within, the substance of the nerve. This tacit convention has been challenged in recent years with the publication of a series of reports suggesting that intraneural needle placement, and indeed injection of local anesthetic, may not necessarily result in detectable clinical injury. In 2004 Sala-Blanch et al described two cases of placement of a catheter within the epineurium of the sciatic nerve, confirmed by computerized tomographic imaging. (8) Both patients demonstrated clinically efficacious blocks without postoperative neurologic deficit. The advent of ultrasound guidance for nerve blocks has likely led to an increase in the recognition of inadvertent intraneural injections. Accidental femoral9 and musculocutaneous (10) intraneural injections have been described, as evidenced by nerve swelling on the ultrasound image, both without lasting neurologic effect. In 2006, Bigeleisen published a series of axillary brachial plexus blocks performed on 22 patients undergoing thumb surgery. (11) Using ultrasound guidance, the authors deliberately placed the needle intraneurally and injected 2 to 3 mL of local anesthetic, which resulted in 72 intraneural injections as evidenced by nerve swelling. Despite the common occurrence of paresthesia or dysesthesia (66 times), none of the patients developed an overt neurologic deficit up to 6 months postoperatively. Similarly, Robards et al studied 24 patients receiving sciatic nerve blocks in the popliteal fossa using both nerve stimulation and ultrasound guidance. (12) The end point for needle advancement was a motor response using a current intensity of 0.2 to 0.5 mA, or an apparent intraneural needle tip location, whichever came first. These investigators found that the motor response could only be obtained upon entry of the needle into the nerve in 83.3% of patients; in the remaining 16.7%, a motor response with a stimulating current of 1.5 mA could not be obtained, even when the needle tip was intraneural. There was no postoperative neurologic dysfunction. Taken together, these studies suggest that an intraneural needle placement with resultant injection of the local anesthetic within internal epineurium does not lead to an imminent neurologic injury. The data by Robards et al, suggest that many nerve blocks without the benefit of ultrasound visualization, have likely resulted in intraneural (intra-epineural) injections. The reason why nerve injury is infrequent is that the vast majority of these injections do not occur within fascicles. Extrafascicular versus Intrafascicular Injections A needle placed within a peripheral nerve can be in one of two locations: within the loose epineurial matrix that surrounds the fascicles or inside a fascicle itself. It is well established that injection of even very small amounts of local anesthetic within the fascicle can lead to widespread axonal degeneration and permanent neural damage in animals, whereas extrafascicular injection does not disrupt the normal nerve architecture. (7,13) Part of this can be explained mechanically because the perineurium, a tough multilayer epithelial sheath, is not easily distensible to compensate to an increase in intrafascicular pressure. Intrafascicular pressure rises on injection and can remain higher than the capillary perfusion pressure longer than the duration of the injection itself, predisposing to neural ischemia and inflammation. (14) Furthermore, pressure curves derived from intrafascicular versus extrafascicular injections in canine sciatic nerves show that a pattern of very high initial injection pressures followed by a sharp drop to baseline is associated with poor outcome and severe neural histologic damage, and may suggest fascicular rupture. (15) In contrast, injections into the compliant epineurial space appear to be associated with a minimal rise in pressure, which can be explained by its loose and accommodating stromal architecture. The risk of an intrafascicular injection differs from site to site in the peripheral nervous system, and it correlates with the cross-sectional fascicle-epineurium ratio. For example, the sciatic nerve at the popliteal fossa contains more nonneural tissue than fascicles in its cross-sectional area, which corresponds with its low incidence of post-PNB neuropathy. (16) By contrast, the brachial plexus at the level of the trunks contains much more neural than connective tissue; a needle entering the nerve here is more likely to encounter a fascicle on its trajectory that may contribute to the disproportionately higher rate of postoperative neuropathy following PNB with interscalene blocks. (17) As peripheral nerves move away from the neuraxis, the ratio of connective tissue to neural tissue within the nerve tends to increase. The brachial plexus elements below the clavicle have a ratio of connective tissue to neural tissue of approximately 2:1, whereas the more proximal trunks and divisions have a ratio of 1:1. (18) Mechanisms of Nerve Injury Following Intraneural Injection Once the perineurium is breached, the spectrum of subsequent injury is wide and multifactorial. Needle Trauma The mechanical disruption of the perineurial sheath may result in injury to the axons and/or the leakage/herniation of endoneural contents. (19) However, the composition of the injectate may play a larger role in the outcome of intrafascicular injection. For example, normal saline injected into fascicles did not cause any damage in one study, suggesting that mere puncture of the perineurium does not necessarily result in clinically overt injury. (13) In contrast, nerve puncture with intravenous cannulae or electroneurography needles has been shown to result in lasting neurologic deficit. (20-22) A variety of cellular changes accompany needle trauma, including alterations in membrane channel expression, activation of signal transduction, neuropeptide production, and an overall increase in excitability at the dorsal horn. (23,24) The effect of the needle size on the likelihood and severity of the injury is controversial, however, smaller needles (24 gauge) may lead to less nerve injury than larger needles (19 gauge). (25) Despite the concern over fascicular puncture, due to their compact nature, fascicles are more likely to escape the advancing needle, rather than be penetrated under normal PNB conditions. Early work by Selander et al in rabbits demonstrated that needle tip characteristics influenced the likelihood of fascicular penetration. (26) This study demonstrated that long-bevel (12-15°) needles were more likely to puncture the fascicle than short-bevel (45°) needles, and resulted in the author advocating for their use during PNB. A more recent study compared blunt (30°) versus sharp (15°) needles by passing these needles through a cadaveric sciatic nerve and examining the nerve microscopically afterward for signs of fascicular damage. (27) Although a total of 134 fascicles were identified as being in contact with the needle tracks, only 4 fascicles were damaged, all of which belonged to the sharp-tip group. These data suggest that a needle passing through a fascicle is more likely only to encounter epineurium and may in fact displace the tough fascicles away from the needle path. Although blunt needles are less likely to enter the fascicle, once penetrated, blunt needles appear to cause a greater degree of injury compared with sharp needles, especially if the sharp needles are oriented with the bevel in the same direction as the nerve fibers (i.e., not cutting transversely across the fibers). (28) Regardless of which needle type or size enters the nerve, a needle insertion into nerve and consequent injection invariably leads to inflammation and cellular infiltration regardless of whether a clinical injury occurs. Toxicity of Local Anesthetics and Additives Although all local anesthetics are potentially neurotoxic, (29) the mechanism remains unclear. Proposed mechanisms include increases in intracellular calcium concentration, disturbance in mitochondrial function, interference with membrane phospholipids, and cell apoptosis. (30-33) The perineurium and blood vessel endothelium serve as a barrier to entry into the fascicle. However, even local anesthetics placed within the epineurium have been shown to cause altered perineural permeability and fascicular edema, leading to compression of the fascicle and reduced neural blood flow. (13,34) This effect appears to be dose dependent. Intraneural administration of local anesthetics exposes the axons to higher concentrations of drug than extraneural application. One study comparing the extraneural, extrafascicular, and intrafascicular administration of ropivacaine 0.75% showed that histologic damage was least severe extraneurally and most severe intrafascicularly. (35) However, even when injected inside the epineurium, others have shown ropivacaine 0.75% to have no adverse effect on functional recovery. (36) Ester local anesthetics such as tetracaine and chloroprocaine were shown in some studies to cause a greater degree of injury than those of the amide group, but recent data have challenged those conclusions. (34,37) What is well known is that the injection of local anesthetics into the fascicle results in widespread and immediate axonal injury. (14) Local anesthetics alone are also capable of decreasing neural blood flow. Lidocaine 2% reduces neural blood flow in rat sciatic nerves by 20-40%, and this difference persists after washout of the local anesthetic solution. (38,39) Increasing concentrations of lidocaine appear to reduce neural blood flow further; the reverse is true for bupivacaine. Altering the concentration of tetracaine appears to have no effect on neural blood flow. Various concentrations of levobupivacaine and ropivacaine have been found to reduce rat sciatic nerve blood flow significantly. (40) Epinephrine is a common adjuvant used to prolong the duration of blockade and to warn of intravascular injection/ absorption. At concentrations of 5 μg/mL and 10 μg/mL, epinephrine reduces neural blood flow by 20% and 35%, respectively. (39) In contrast, at lower concentrations (2.5 μg/mL), neural blood flow increases by 20% transiently before returning to baseline, suggesting that at lower concentrations the β-adrenergic effects predominate. The effects of combining lidocaine and epinephrine are additive: A solution of 2% lidocaine plus 5 μg/mL of epinephrine reduced neural blood flow by 80%. (38) The clinical significance of the effects of various local anesthetics and additives is unknown. The experimental data must be weighed within the context of clinical practice; countless of nerve blocks of blocks are performed in daily practice using a combination of local anesthetic and epinephrine with no neurologic consequences. This reinforces the hypothesis that nerve injury is multifactorial, and one theoretical aspect may be insufficient to cause injury consistently. Prevention of Peripheral Nerve Injury Several techniques have been advocated to enhance safety during the performance of PNBs. The merits of each technique in preventing nerve injury are addressed individually. Pain on Injection Pain on injection has traditionally been taught as a reliable and effective means to guard against intraneural injection because intraneural injections are thought to be exquisitely painful. However, there are multiple problems with this logic. First, pain is notoriously difficult to evaluate in terms of intensity and quality. Consequently, differentiating between a benign, commonly present discomfort during injection of local anesthetic (pressure paresthesia) and that of intrafascicular injection can be elusive. Second, various patient conditions (e.g., diabetes mellitus, peripheral neuropathy) and premedication may interfere with pain perception. Third, there appears to be little evidence that pain on injection is either sensitive or specific. Fanelli et al conducted a prospective study of nearly 4000 patients receiving multiple-injection PNBs and found that the overall rate of neurologic complications was 1.7%, independent of whether the patient reported a paresthesia or not. (41) In other words, it does not appear to matter whether the patient reports a paresthesia or not they have an equal likelihood of postoperative neuropathy. Bigeleisen's report of 72 intraneural injections was associated with 66 reports of paresthesia or dysesthesia, yet none of the patients had neurologic complications, suggesting the symptom itself has a low specificity for complications. (11) Fourth, the nature of nerve injury might preclude its use as a useful monitor: By the time a patient registers pain, communicates it to the anesthesiologist, and the injection is halted, the damage is likely to have been done. Because a fraction of a milliliter is sufficient to cause irreversible fascicular damage, the patient's subjective symptom may be too late. (7,15) Finally, there are situations in which performing PNBs in an asleep/ heavily sedated/blocked patient might be the safest approach, for example, pediatric cases, mentally incompetent patients, the traumatically injured, patients needing rescue or repeat blocks, and so on. The use of more objective monitors, as listed here, may provide increased confidence that an intrafascicular injection can be avoided when compared with a subjective patient symptom. Electrical Nerve Stimulation Electrical stimulation is a means to locate nerves but also may be used to rule out an intraneural (intrafascicular) location of the needle. Voelckel et al demonstrated that sciatic blocks in pigs performed with a motor response at These studies suggest that, although neurostimulation techniques may not be a highly sensitive method of detecting intraneural needle tip position (i.e., high current intensities may still be required to elicit motor responses even with intra-epineural needle placement), neurostimulation has a high specificity for identifying intraneural needle tip placement (i.e., motor response at ≤0.2 mA obtained only with intraneural needle tip location) Based on the cumulative experimental and clinical data, using no motor response at Ultrasonography Ultrasound guidance is theoretically an attractive means of preventing intraneural injection due to real-time imaging of the needle and nerve. Indeed, nerve swelling visualized on the sonographic image appears to represent true histologic intraneural injection as evaluated by the presence of India ink staining within the epineurium. (44,46) However, the clinical implications of this are also unclear because nerve swelling and even histologic changes associated with nerve injury appear not to result in detectable neurologic deficit in pigs, although there may be subtle changes that cannot be assessed by the evaluators. (47) Ultrasound guidance may not be a substantially effective means of preventing nerve injury. The reliability of ultrasound to keep the needle tip extraneural depends largely on the skill of the operator and the imaging characteristics of the needle and tissue. Several case reports of accidental nerve (and vascular) puncture despite the use of ultrasound guidance highlight the fact that ultrasound monitoring is not a fail-proof method of avoiding neurologic (and other mechanical) complications. (9,10,48) Furthermore, at the present time the resolution of the sonographic image is such that it would be impossible to tell if the needle tip was intrafascicular or extrafascicular, which is the critical anatomic differentiation to make to avoid nerve injury. Finally, as is the case with paresthesia, by the time the nerve is seen swelling on the image, the damage may have already been done if the injection is made with the needle tip inside the fascicle. injection pressure Monitoring The crux of the intraneural injection problem thus far appears to lie in the avoidance of penetrating the perineurium and entering the fascicle. The presence of a high opening injection pressure (>20 psi) in a canine model is a very sensitive (if not highly specific) sign of intrafascicular needle tip placement, whereas extrafascicular needle tip placement is associated with low (20 psi) pressures, whereas high pressures were absent during extraneural injection. (49) More importantly, high pressure injection was associated with neurologic deficits and severe axonal damage after the block, in contrast to normal neurologic and histologic findings following any low-pressure injection (extra or intraneural). Indeed, PNBs associated with high injection pressure, despite a lack of paresthesia, have been reported to result in permanent neurologic injury. (50) Although objective injection pressure monitoring and documentation has not yet been universally adopted, assessment of resistance to injection is a standard clinical practice. Unfortunately, clinical data on the role of injection pressure monitoring may never become available as it would be unethical to randomize patients to receive a high versus low pressure nerve block injection. However, the available clinical and experimental evidence points that injection pressure is useful in detecting needle-nerve contact (Table 1). Given this, safe practice should include the objective assessment of the resistance to injection with most single injection peripheral nerve blocks. An assessment of injection resistance is often assessed using a "syringe-hand-feel" technique. However, it has been well documented (in at least two models) that practitioners are unable to gauge injection pressure by using a syringe-hand-feel subjective technique. (51,52) Therefore, if monitoring of resistance to injection is to have clinical merit, objective monitoring of injection pressure should be used to standardize the injection force. This may be achieved by use of commercially available inline devices or with the use of a "compressed air injection technique." (53) One shortcoming of injection pressure monitoring is that injection pressure is highly sensitive but lacks specificity. In other words, absence of high injection pressure appears to effectively rule out an intrafascicular injection. However, high injection pressure also can be caused by PNB needle obstruction, attempted injection into a tendon, or tissue compression caused by the ultrasound transducer. Future Directions The regional anesthesia community is witnessing the beginning of a paradigm shift in the thinking surrounding intraneural injection during PNB. Clearly they can be performed safely in certain patients. The question is: Should they? Even intranueral extrafascicular injection of local anesthetic often results in histologic evidence of inflammation in animal experiments. However, intraneural extrafascicular injection in patients often does not result in symptoms. The risk may be different in patients who have preexisting or subclinical neuropathy. It is important to note that the studies demonstrating safe intraneural injections in humans deliberately excluded patients with preexisting neuropathy. Table 1 Deliberate injection of local anesthetic into peripheral nerves and plexuses is controversial with regards to its safety and clinical advantages. Such injections in distal nerves may be more forgiving, owing to their increased ratio of nonneural tissue to neural tissue. In particular, injuries to the sciatic nerve at the popliteal fossa appear to be uncommon following intraneural injection or intraneural catheter placement. (54,55) In fact, it has been noted that intraneural injection of local anesthetic in the popliteal sciatic nerve leads to a rapid onset of sensory and motor blockade, without complications. (55) Some practitioners now routinely attempt to place the needle tip within the epineurium at this location, in an attempt to hasten onset, improve block success, and decrease the total amount of local anesthetic required. However, this practice should be done only with a combination of objective monitoring because the need to remain extrafascicular is paramount. One of the challenges is to elucidate the precise factors that provide for a safe intraneural injection, whether anatomic (popliteal sciatic versus subgluteal), technological (injection pressure monitoring, improved resolution of ultrasound imaging), educational (improved training), or otherwise. More clinical research is needed to clarify the safety of intraneural injection in various nerves such as the femoral nerve, and distal nerves of the upper and lower limbs. This should be undertaken with care and with proper safeguards to prevent penetration of the perineurium. Lastly, intraneural injection may allow for reduction in the volume and/or concentration of local anesthetic required for effective nerve block. (54) This is a worthwhile avenue to explore, both in terms of the implications for nerve injury and for reducing the potential for systemic local anesthetic toxicity. 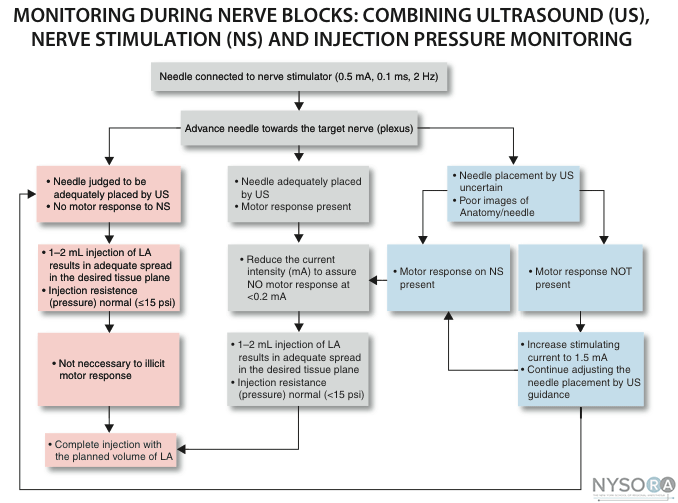 Practical Management of Postoperative Neuropathy Despite the best precautions, a postoperative sensory or motor deficit that outlasts the expected duration of the PNB may occur. It is important to note that the vast majority of neuropathies resolve spontaneously, and patient reassurance is vital. (36) Processes that are either evolving (i.e. compartment syndrome) or are reparable (i.e. surgical transection of a nerve) should be ruled out. Here are a few principles to bear in mind when managing a postoperative neuropathy: 1. Good communication is essential, both from a patient care and medicolegal standpoint. 2. Approximately 95% of postoperative sensory changes will resolve within 4-6 weeks, and most of these will occur during the first week. 99% of sensory changes will resolve within the first year. 3. Early diagnosis of postoperative peripheral nerve injury can be challenging due to: 4. Neuropathies can also be caused by prolonged tourniquets, casting, excessive intraoperative traction, or a misplaced surgical clip. Early involvement of the surgical team is prudent. 5. In general, the presence of motor deficits is an ominous sign, and a referral to a neurologist and/or neurosurgeon is indicated. 6. Neuropathies that are evolving, and those that are severe/ complete should be seen immediately by a neurologist and/or neurosurgeon. Referral for electrophysiologic testing may be indicated when the symptoms are not purely sensory, or when the neuropathy is severe and/or long-lasting. Studies performed usually consist of the following: 1. Electromyography. This is undertaken to determine which muscle units are affected by a denervation lesion. Small needle electrodes are placed in various muscles and the pattern of electrical activity both at rest and with contraction is analyzed. The results can help to localize a lesion, and, depending on the pattern, suggest a time frame for the injury. 2. Nerve conduction studies. In these tests, a device similar to the peripheral nerve stimulator used by anesthesiologists to monitor the degree of neuromuscular blockade is attached over various nerves in the affected area. A characteristic waveform is generated following stimulation of the nerve, which may allow the neurologist to pinpoint a conduction block. The optimal timing of these tests depends on the indication. An exam within 2-3 days of the onset of injury may give information regarding the completeness of the lesion (and therefore prognosis), as well as clues about the duration of the lesion, which often has medicolegal ramifications, particularly if the lesion is deemed to predate the nerve block or surgical procedure. As such, this can be seen as a "baseline" exam. More information is obtained at approximately 4 weeks post-injury, when the electrophysiologic changes have had an opportunity to evolve more fully. A practical algorithm for the management of postoperative neuropathy is shown in Figure 3.
|
| 12/19/2015(+ 2016 Dates) | |
| 01/27/2016 | |
| 03/17/2016 | |
| 04/20/2016 | |
| 09/24/2016 | |
| 10/01/2024 |
admin
- Anatomy of Nerve Blockade
- Ultrasound-Guided Interscalene Brachial Plexus Block
- Ultrasound-Guided Popliteal Block
- Ultrasound-Guided Supraclavicular Brachial Plexus Block
- Ultrasound-Guided Fascia Iliaca Block
- Ultrasound-Guided Femoral Nerve Block
- Neurologic Complications of Peripheral Nerve Blocks
- Ultrasound-Guided Interscalene Brachial Plexus Block
- Ultrasound-Guided Axillary Brachial Plexus Block
![[advertisement] gehealthcare](../files/banners/banner1_250x600/GEtouch(250X600).gif)

![[advertisement] concertmedical](../files/bk-nysora-ad.jpg)


Post your comment