Spinal Anesthesia
|
Introduction with General Considerations & Brief History Carl Koller, an ophthalmologist from Vienna, first described the use of topical cocaine for analgesia of the eye in 1884.[1] William Halsted and Richard Hall, surgeons at Roosevelt Hospital in New York City, took the idea of local anesthesia a step further by injecting cocaine into human tissues and nerves in order to produce anesthesia for surgery.[2] James Leonard Corning, a neurologist in New York City, described the use of cocaine for spinal anesthesia in 1885.[3] Since Corning was a frequent observer at Roosevelt Hospital, the idea of using cocaine in the subarachnoid space may have come from observing Halsted and Hall performing cocaine injections. Corning first injected cocaine intrathecally into a dog and within a few minutes the dog had marked weakness in the hindquarters.[4] Next, Corning injected cocaine into a man at the T11-T12 interspace into what he thought was the subarachnoid space. Since Corning did not notice any effect after 8 min, he repeated the injection. Ten minutes after the second injection, the patient complained of sleepiness in his legs, but was able to stand and walk. Because Corning made no mention of cerebrospinal fluid (CSF) efflux, most likely he inadvertently gave an epidural rather than a spinal injection to the patient. Dural puncture was described by Essex Wynter in 1891[5] followed shortly by Heinrich Quincke 6 months later.[6] Augustus Karl Gustav Bier, a German surgeon, used cocaine intrathecally on six patients for lower extremity surgery in 1898.[7,8] In true scientific fashion, Bier decided to experiment on himself and developed a postdural puncture headache (PDPH) for his efforts. His assistant, Dr. Otto Hildebrandt, volunteered to have the procedure performed after Bier was unable to continue due to the PDPH. After injection of spinal cocaine into Hildebrandt, Bier conducted experiments on the lower half of Hildebrandt's body. Bier described needle pricks and cigar burns to the legs, incisions on the thighs, avulsion of pubic hairs, strong blows with an iron hammer to the shins, and torsion of the testicles. Hildebrandt reported minimal to no pain during the experiments; however, afterward he suffered nausea, vomiting, PDPH, and bruising and pain in his legs. Bier attributed the PDPH to loss of CSF and felt the use of small-gauge needles would help prevent the headache.[9] Dudley Tait and Guido Caglieri performed the first spinal anesthetic in the United States in San Francisco in 1899. Their studies included cadavers, animals, and live patients in order to determine the benefits of lumbar puncture, especially in the treatment of syphilis. Tait and Caglieri injected mercuric salts and iodides into the CSF, but worsened the condition of one patient with tertiary syphilis.[10] Rudolph Matas, a vascular surgeon in New Orleans, described the use of spinal cocaine on patients and possibly was the first to use morphine in the subarachnoid space.[11,12] Matas also described the complication of death after lumbar puncture. Theodore Tuffier, a French surgeon in Paris, studied spinal anesthesia and reported on it in 1900. Tuffier felt that cocaine should not be injected until cerebrospinal fluid was recognized.[13] Tuffier taught at the University of Paris at the same time that Tait was a medical student there and most likely was one of Tait's mentors. Tuffier's demonstrations in Paris helped popularize spinal anesthesia in Europe. Arthur Barker, a professor of surgery at the University of London, reported on the advancement of spinal techniques in 1907, including the use of a hyperbaric spinal local anesthetic, emphasis of sterility, and ease of midline over paramedian dural puncture.[14] Advancement of sterility and the investigation of decreases in blood pressure after injection helped make spinal anesthesia safer and more popular. Gaston Labat was a strong proponent of spinal anesthesia in the United States and performed early studies on the effects of Trendelenburg position on blood pressure after spinal anesthesia.[15] George Pitkin attempted to use a hypobaric local anesthetic to control the level of spinal block by mixing procaine with alcohol.[16] Lincoln Sise, an anesthesiologist at the Lahey Clinic in Boston, used Barker's technique of hyperbaric spinal anesthesia with both procaine and tetracaine.[17-19] Spinal anesthesia became more popular as new developments occurred, including the introduction of saddle block anesthesia by Adriani and Roman-Vega in 1946.[20] The height of spinal anesthesia's popularity in the United States occurred in the 1940s, but fears of neurologic deficits and complications caused anesthesiologists to discontinue the use of spinal anesthesia. The development of novel intravenous anesthetic agents and neuromuscular blockers coincided with the decreased use of spinal anesthesia. In 1954 Dripps and Vandam described the safety of spinal anesthetics in more than 10,000 patients,[21] and spinal anesthesia was revived. The early development of spinal needles paralleled the early development of spinal anesthesia. Corning chose a gold needle that had a short bevel point, flexible cannula, and set screw that fixed the needle to the depth of dural penetration. Corning also used an introducer for the needle, which was right-angled. Quincke used a beveled needle that was sharp and hollow. Bier developed his own sharp needle that did not require an introducer. The needle was larger bore (15-gauge or 17-gauge) with a long, cutting bevel. The main problems with Bier's needle were pain on insertion and the loss of local anesthetic due to the large hole in the dura after dural puncture. Barker's needle did not have an inner cannula, was made of nickel, and had a sharp, medium length bevel with a matching stylet. Labat developed an unbreakable nickel needle that had a sharp, short-length bevel with a matching stylet. Labat believed that the short bevel minimized damage to the tissues when inserted into the back. Herbert Greene realized that loss of CSF was a major problem in spinal anesthesia and developed a smooth tip, smaller gauge needle that resulted in a lower incidence of PDPH.[22] Barnett Greene described the use of a 26-gauge spinal needle in obstetrics with a decreased incidence of PDPH.[23] The Greene needle was very popular until the introduction of the Whitacre needle. Hart and Whitacre used a pencil-point needle to decrease PDPH from 5-10% to 2%.[24] Sprotte modified the Whitacre needle and published his trial of over 34,000 spinal anesthetics in 1987.[25] Modifications of the Sprotte needle occurred the 1990s to produce the needle that is in use today.[26] Spinal anesthesia has progressed greatly since 1885 and is used successfully in a number of different clinical situations. However, anatomy, choice of local anesthetic, physiologic effects of spinal anesthesia, patient positioning, and the approach to spinal anesthesia must all be considered. The patient should be educated about the possible side effects and complications that can occur from performing a spinal anesthetic in order to obtain informed consent before the procedure. If all of these factors are conducive for the patient to receive a spinal anesthetic, care must be taken to prevent complications. Learning how to perform spinal anesthesia is an invaluable skill that all anesthesiologists should have in their armamentarium. Contraindications to Spinal Anesthesia There are absolute and relative contraindications to spinal anesthesia. The only absolute contraindications include patient refusal, infection at the site of injection, hypovolemia, indeterminate neurologic disease, coagulopathy, and increased intracranial pressure, except in cases of pseudotumor cerebri. Relative contraindications include sepsis distinct from the anatomic site of puncture (e.g., chorioamnionitis or lower extremity infection) and unknown duration of surgery. In the latter case, if the patient is on antibiotics and the vital signs are stable, spinal anesthesia may be considered.
Prior to placing a spinal anesthetic, the anesthesiologist should examine the patient's back to look for any signs of infection, which may increase the risk of meningitis. Preoperative shock or hypovolemia increases the risk of hypotension after placement of a spinal anesthetic. High intracranial pressure increases the risk of uncal herniation when CSF is lost through the needle. If intracranial pressure rises after injection of the spinal anesthetic, brain herniation can occur. Coagulation abnormalities increase the risk of hematoma formation. It is important to communicate with the surgeon to determine the amount of time needed to complete the operation before inducing spinal anesthesia. If the duration of surgery is unknown, the spinal anesthetic given may not be long enough to cover the surgery. Knowing the duration of surgery helps the anesthesiologist determine the local anesthetic that will be used, addition of spinal adjuncts such as epinephrine, and whether a spinal catheter will be necessary. Another consideration when performing spinal anesthesia is the site of surgery, since surgery above the umbilicus would be difficult to cover with a spinal as the sole technique. Performing spinal anesthesia in patients with neurologic diseases, such as multiple sclerosis, is controversial due to in vitro experiments that determine that demyelinated nerves are more susceptible to local anesthetic toxicity. However, no clinical study has convincingly demonstrated that spinal anesthesia worsens such neurologic diseases. Indeed, with the knowledge that pain, stress, fever, and fatigue exacerbate these diseases, a stress-free central neuraxial block may be preferred for surgery.[27-31] Cardiac disease when sensory levels above T6 are required is a relative contraindication to spinal anesthesia.[32,33] Certain cardiac diseases, such as aortic stenosis, once considered to be an absolute contraindication for spinal anesthesia, may now incorporate a carefully conducted spinal anesthetic into their anesthetic care.[34-36] Severe deformities of the spinal column can increase the difficulty in placing a spinal anesthetic. Arthritis, kyphoscoliosis, and previous lumbar fusion surgery all factor into the ability of the anesthesiologist to performa spinal anesthetic. It is essential to examine the patient's back to determine any anatomic abnormality before attempting a spinal anesthetic. Functional Anatomy Of Spinal Blockade In reviewing the functional anatomy of spinal blockade, an intimate knowledge of the spinal column, spinal cord, and spinal nervesmust be present. This chapter reviews briefly the curves of the vertebral column, the ligaments of the spinal column, membranes and length of the spinal cord, and passage of the spinal nerves from the spinal cord. The vertebral column consists of 33 vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 4 coccygeal segments. The vertebral column usually contains three curves. The cervical and lumbar curves are convex anteriorly, and the thoracic curve is convex posteriorly. The vertebral column curves, alongwith gravity, baricity of local anesthetic, and patient position, influence the spread of local anesthetics in the subarachnoid space. Figure 1 depicts the spinal column, vertebrae, and intervertebral discs and foramina. 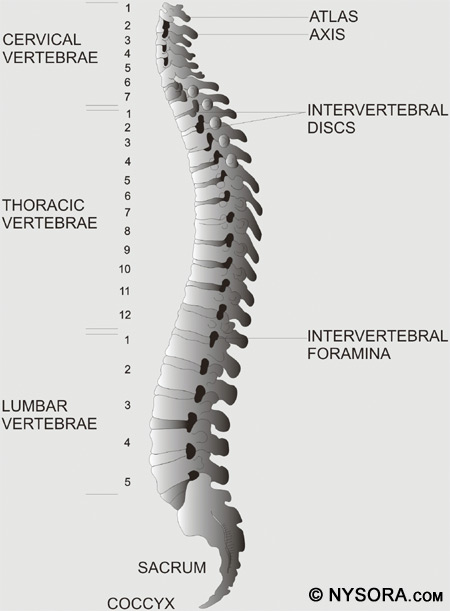
Figure 1: The spinal column is seen from a lateral view. All of the vertebrae, intervertebral discs, and intervertebral foraminae are shown.
Five ligaments hold the spinal column together. The supraspinous ligaments connect the apices of the spinous processes from the seventh cervical vertebra (C7) to the sacrum. The supraspinous ligament is known as the ligamentum nuchae in the area above C7. The interspinous ligaments connect the spinous processes together. The ligamentum flavum, or yellow ligament, connects the laminae above and below together. Finally, the posterior and anterior longitudinal ligaments bind the vertebral bodies together. Figure 2 shows a cross section of the spinal canal with the ligaments, vertebral body, and spinous processes. 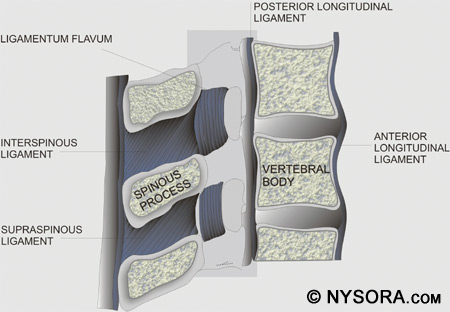
Figure 2: A cross section of the spinal canal is shown with the ligaments, vertebral body, and spinous processes. The three membranes that protect the spinal cord are the dura mater, arachnoid mater, and pia mater. The dura mater, or tough mother, is the outermost layer. The dural sac extends to the second sacral vertebra (S2). The arachnoid mater is the middle layer, and the subdural space lies between the dural mater and arachnoid mater. The arachnoid mater, or cobweb mother, also ends at S2, like the dural sac. The pia mater, or soft mother, clings to the surface of the spinal cord and ends in the filum terminale, which helps to hold the spinal cord to the sacrum. The space between the arachnoid and pia mater is known as the subarachnoid space, and spinal nerves run in this space, as does CSF. Figure 3 depicts the spinal cord, dorsal root ganglia and ventral rootlets, spinal nerves, sympathetic trunk, rami communicantes, and pia, arachnoid, and dura mater. 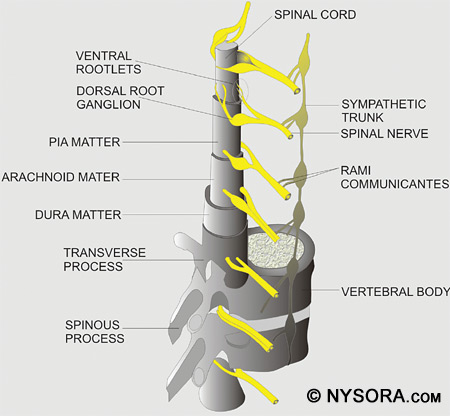
Figure 3: The spinal cord is shown along with the dorsal root ganglia and ventral rootlets, spinal nerves, sympathetic trunk, rami communicantes, and pia, arachnoid, and dura mater. When performing a spinal anesthetic using the midline approach, the layers of anatomy that are traversed (from posterior to anterior) are skin, subcutaneous fat, supraspinous ligament, interspinous ligament, ligamentum flavum, dura mater, subdural space, arachnoid mater, and finally the subarachnoid space. When the paramedian technique is applied, the spinal needle should traverse the skin, subcutaneous fat, ligamentum flavum, dura mater, subdural space, arachnoid mater, and then pass into the subarachnoid space. The length of the spinal cord varies according to age. In the first trimester, the spinal cord extends to the end of the spinal column, but as the fetus ages, the vertebral column lengthens more than the spinal cord. At birth, the spinal cord ends at approximately L3 and in the adult, the cord ends at approximately L1 with 30% of people having a cord that ends at T12 and 10% at L3. Figure 4 shows a cross section of the lumbar vertebrae and spinal cord. The position of the conus medullaris, cauda equina, termination of the dural sac, and filum terminale are shown. A sacral spinal cord in an adult has been reported, though this is extremely rare.[37] The length of the spinal cord must always be kept in mind when a neuraxial anesthetic is performed, as injection into the cord can cause great damage and result in paralysis.[38] 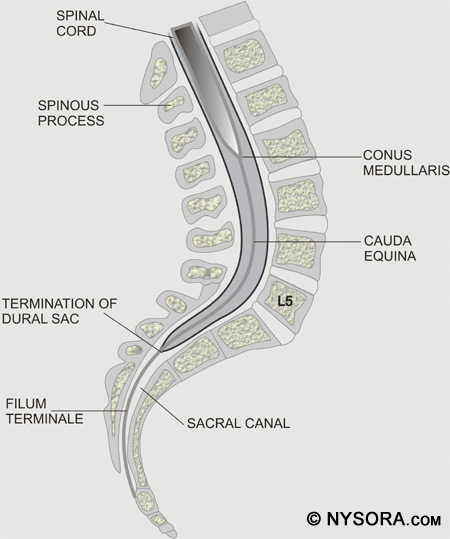
Figure 4: A cross section of the lumbar vertebrae and spinal cord. The position of the conus medullaris, cauda equina, termination of the dural sac, and filum terminale are shown. Spinal nerves in the cervical region are named according to the upper cervical vertebral body from which they exit. However, the eighth cervical nerve exits from below the seventh cervical vertebral body, and this method of naming continues in the thoracic and lumbar regions. The spinal nerve roots and spinal cord serve as the target sites for spinal anesthesia. Surface Anatomy When preparing for spinal anesthetic blockade, it is important to find landmarks on the patient. The iliac crests usually mark the interspace between the fourth and fifth lumbar vertebrae, and a line can be drawn between them to help locate this interspace. Care must be taken to feel for the soft area between the spinous processes to locate the interspace. Depending on the level of anesthesia necessary for the surgery and the ability to feel for the interspace, the L3-4 interspace or the L4-5 interspace can be used to introduce the spinal needle. Because the spinal cord ends at the L1 to L2 level, it would not bewise to attempt spinal anesthesia at or above this level.
It would be incomplete to discuss surface anatomy without mentioning the dermatomes that are important for spinal anesthesia. A dermatome is an area of skin innervated by sensory fibers from a single spinal nerve. The tenth thoracic (T10) dermatome corresponds to the umbilicus, the sixth thoracic (T6) dermatome the xiphoid, and the fourth thoracic (T4) dermatome the nipples. Figure 5 illustrates the dermatomes of the human body. To achieve surgical anesthesia for a given procedure, the extent of spinal anesthesia must reach a certain dermatomal level. Dermatomal levels of spinal anesthesia for common surgical procedures are listed in Table 1. 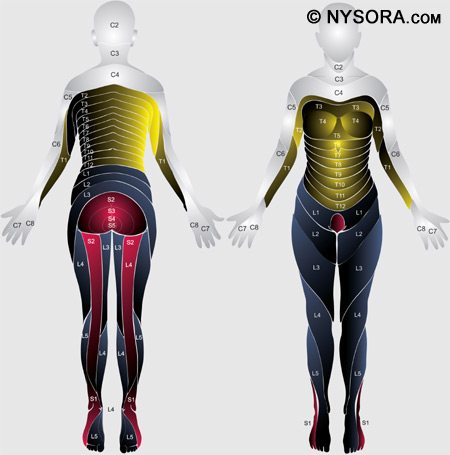
Figure 5: The dermatomes of the human body.
Pharmacology The choice of local anesthetic is based on potency of the agent, onset and duration of anesthesia, and side effects of the drug. Two distinct groups of local anesthetics are used in spinal anesthesia, esters and amides, which are characterized by the bond that connects the aromatic portion and the intermediate chain. Esters contain an ester link between the aromatic portion and the intermediate chain, and examples include procaine, chloroprocaine, and tetracaine. Amides contain an amide link between the aromatic portion and the intermediate chain, and examples include bupivacaine, ropivacaine, etidocaine, lidocaine, mepivacaine, and prilocaine. Although metabolism is important for determining activity of local anesthetics, lipid solubility, protein binding, and pKa also influence activity.[39]
Lipid solubility relates to the potency of local anesthetics. Low lipid solubility indicates that higher concentrations of local anesthesia must be given to obtain nerve blockade. High lipid solubility produces anesthesia at low concentrations. Protein binding affects the duration of action of a local anesthetic. Higher protein binding results in longer duration of action. The pKa of a local anesthetic is the pH at which ionized and nonionized forms are present equally in solution, which is important because the nonionized form allows the local anesthetic to diffuse across the lipophilic nerve sheath and reach the sodium channels in the nerve membrane. The onset of action relates to the amount of local anesthetic available in the base form. Most local anesthetics follow the rule that the lower the pKa, the faster the onset of action and vice versa. Pharmacokinetics of Local Anesthetics in the Subarachnoid Space Pharmacokinetics of local anesthetics includes uptake and elimination of the drug. Four factors play a role in the uptake of local anesthetics from the subarachnoid space into neuronal tissue, (1) concentration of local anesthetic in CSF, (2) surface area of nerve tissue exposed to CSF, (3) lipid content of nerve tissue, and (4) blood flow to nerve tissue.[40,41] The uptake of local anesthetic is greatest at the site of highest concentration in the CSF and is decreased above and below this site. As discussed previously, uptake and spread of local anesthetics after spinal injection are determined by multiple factors including dose, volume, and baricity of local anesthetic and patient positioning. Both the nerve roots and the spinal cord take up local anesthetics after injection into the subarachnoid space. The more surface area of the nerve root exposed, the greater the uptake of local anesthetic.[42-45] The spinal cord has two mechanisms for uptake of local anesthetics. The first mechanism is by diffusion from the CSF to the pia mater and into the spinal cord, which is a slow process. Only the most superficial portion of the spinal cord is affected by diffusion of local anesthetics. The second method of local anesthetic uptake is by extension into the spaces of Virchow-Robin, which are the areas of pia mater that surround the blood vessels that penetrate the central nervous system. The spaces of Virchow-Robin connect with the perineuronal clefts that surround nerve cell bodies in the spinal cord and penetrate through to the deeper areas of the spinal cord. Figure 6 is a representation of the periarterial Virchow-Robin spaces around the spinal cord. 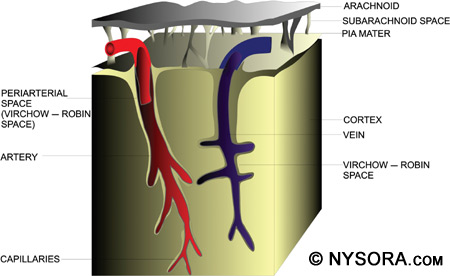
Figure 6: A representation of the periarterial Virchow–Robin spaces around the spinal cord.
Lipid content determines uptake of local anesthetics. Heavily myelinated tissues in the subarachnoid space contain higher concentrations of local anesthetics after injection. The higher the degree of myelination, the higher the concentration of local anesthetic, as there is a high lipid content in myelin. If an area of nerve root does not contain myelin, an increased risk of nerve damage occurs in that area.[46] Blood flow determines the rate of removal of local anesthetics from spinal cord tissue. The faster the blood flow in the spinal cord, the more rapid the anesthetic is washed away. This may partly explain why the concentration of local anesthetics is greater in the posterior spinal cord than in the anterior spinal cord, even though the anterior cord is more readily accessed by the Virchow-Robin spaces. After a spinal anesthetic is administered, blood flow may be increased or decreased to the spinal cord, depending on the particular local anesthetic administered, e.g., tetracaine increases cord flow but lidocaine and bupivacaine decrease it, which affects elimination of the local anesthetic.[47-49] Elimination of local anesthetic from the subarachnoid space is by vascular absorption in the epidural space and the subarachnoid space. Local anesthetics travel across the dura in both directions. In the epidural space, vascular absorption can occur, just as in the subarachnoid space. Vascular supply to the spinal cord consists of vessels located on the spinal cord and in the pia mater. Because vascular perfusion to the spinal cord varies, the rate of elimination of local anesthetics varies.[40] Distribution The distribution and decrease in concentration of local anesthetics is based on the area of highest concentration, which can be independent of the injection site. Many factors affect the distribution of local anesthetics in the subarachnoid space. Table 2 lists some of these factors.[50] The three most important factors for determining spread of local anesthesia in the subarachnoid space are baricity of the local anesthetic solution, position of the patient during and just after injection, and dose of the anesthetic injected.
Baricity plays an important role in determining the spread of local anesthetic in the spinal space and is equal to the density of the local anesthetic divided by the density of the CSF at 37â—¦C.[51-58] Local anesthetics can be hyperbaric, hypobaric, or isobaric when compared to CSF, and baricity is the main determinant of how the local anesthetic is distributed when injected into the CSF. Table 3 compares the density, specific gravity, and baricity of different substances and local anesthetics.[50,51,53,59,60]
Hypobaric solutions are less dense than CSF and tend to rise against gravity. Isobaric solutions are as dense as CSF and tend to remain at the level at which they are injected. Hyperbaric solutions are more dense than CSF and tend to follow gravity after injection. Hypobaric solutions have a baricity of less than 1.0 relative to CSF and are usually made by adding distilled sterile water to the local anesthetic. Tetracaine, dibucaine, and bupivacaine have all been used as hypobaric solutions in spinal anesthesia. Patient positioning is important after injection of a hypobaric spinal anesthetic because it is the first few minutes that determine the spread of anesthesia. If the patient is in Trendelenburg position after injection, the anesthetic will spread in the caudal direction and if the patient is in reverse Trendelenburg position, the anesthetic will spread cephalad after injection. If a procedure were to be performed in the perineal or anal area in the prone, jackknife position, a hypobaric spinal anesthetic would be an excellent choice to avoid repositioning the patient after injection.
The baricity of isobaric solutions is equal to 1.0. Tetracaine and bupivacaine have both been used with success for isobaric spinal anesthesia, and patient positioning does not affect spread of the local anesthetic, unlike the case with hyperbaric or hypobaric solutions. Injection can be made in any position, and then the patient can be placed into the position necessary for surgery. Gravity dose not play a role in the spread of isobaric solutions, unlike with hypo- or hyperbaric local anesthetics. Hyperbaric solutions in spinal anesthesia have baricity greater than 1.0. A local anesthetic solution can be made hyperbaric by adding dextrose or glucose. Bupivacaine, lidocaine and tetracaine have all been used as hyperbaric solutions in spinal anesthesia. Patient positioning affects the spread of the anesthetic. A patient in Trendelenburg position would have the anesthetic travel in a cephalad direction and vice versa. Dose and volume both play a role in the spread of local anesthetics after spinal injection, although dose has been shown to be more important than volume.[61] Concentration of local anesthetic before injection has no bearing on distribution because after injection, due to the mixing of the CSF and local anesthetic, there is a new concentration. Effects of the Volume of the Lumbar Cistern on Block Height CSF is produced in the brain at 0.35 mL/min and fills the subarachnoid space. This clear, colorless fluid has an approximate adult volume of 150 mL, half of which is in the cranium and half in the spinal canal. However, CSF volume varies considerably, and decreased CSF volume can result from obesity, pregnancy, or any other cause of increased abdominal pressure.[62] This is partly due to compression of the intervertebral foramen which displaces the CSF. Predictability of CSF volume is difficult with weight being the main determinant on physical examination. Multiple factors affect the distribution of local anesthesia after spinal blockade,[50] one being CSF volume. Carpenter showed that lumbosacral CSF volume correlated with peak sensory block height and duration of surgical anesthesia.[63] Higuchi found that CSF density is related to peak sensory block level, and lumbosacral CSF volume correlates to peak sensory block level, and onset and duration of motor block.[64] However, due to thewide variability in CSF volume the ability to predict the level of the spinal blockade after local anesthetic injection is very poor, even if body mass index (BMI) is calculated and used. Effects of the Volume of the Lumbar Cistern on Block Height CSF is produced in the brain at 0.35 mL/min and fills the subarachnoid space. This clear, colorless fluid has an approximate adult volume of 150 mL, half of which is in the cranium and half in the spinal canal. However, CSF volume varies considerably, and decreased CSF volume can result from obesity, pregnancy, or any other cause of increased abdominal pressure.[62] This is partly due to compression of the intervertebral foramen which displaces the CSF. Predictability of CSF volume is difficult with weight being the main determinant on physical examination.
Multiple factors affect the distribution of local anesthesia after spinal blockade,[50] one being CSF volume. Carpenter showed that lumbosacral CSF volume correlated with peak sensory block height and duration of surgical anesthesia.[63] Higuchi found that CSF density is related to peak sensory block level, and lumbosacral CSF volume correlates to peak sensory block level, and onset and duration of motor block.[64] However, due to thewide variability in CSF volume the ability to predict the level of the spinal blockade after local anesthetic injection is very poor, even if body mass index (BMI) is calculated and used. Local Anesthetics Cocaine was the first spinal anesthetic used, and procaine and tetracaine soon followed. Spinal anesthesia performed with lidocaine, bupivacaine, tetracaine, mepivacaine, and ropivacaine have been successful. Some of the more common local anesthetics used for spinal anesthesia will be discussed in this portion of the chapter. As the use of spinal anesthesia becomes more popular, there is more interest in medications that produce anesthesia and analgesia while limiting side effects. A variety of medications, including vasoconstrictors, opioids, α2-adrenergic agonists, and acetylcholinesterase inhibitors, have been added to spinal medications to assist in analgesia while reducing the amount of local anesthetics required for anesthesia. Lidocaine was first used as a spinal anesthetic in 1945, and it has been one of the most widely used spinal anesthetics since. Onset of anesthesia occurs in 3 to 5 min with a duration of anesthesia that lasts for 1 to 1.5 h. Lidocaine spinal anesthesia should be used for short to intermediate length operating room cases, and the most common ampule is 5% lidocaine in 7.5% dextrose. One drawback of lidocaine has been the association with transient neurologic symptoms (TNS), which presents as low back pain and lower extremity dysesthesias with radiation to the buttocks, thighs, and lower limbs after recovery from spinal anesthesia. TNS occurs in about 14% of patients receiving lidocaine spinal anesthesia.[65-67] Because of the risk of TNS associated with lidocaine, other intermediate-acting local anesthetics are being studied to see if their association with TNS may be less. Bupivacaine is a viable alternative to lidocaine for spinal anesthesia and has been used frequently with very little incidence of TNS.[68-70] Onset of anesthesia occurs in to 8 min with a duration of anesthesia that lasts from 210 to 240 min; thus it is appropriate for intermediate to long operating room cases. For outpatient spinal anesthesia, small doses of bupivacaine are recommended to avoid prolonged discharge time due to inability to void. Bupivacaine has replaced lidocaine as the most commonly used spinal local anesthetic in the United States. Bupivacaine is often packaged as 0.75% in 8.25% dextrose. Other forms of spinal bupivacaine include 0.5% with or without dextrose and 0.75% without dextrose. Tetracaine has an onset of anesthesia within 3 to 5 min and a duration of 210 to 240 min, and like bupivacaine, is used for cases that are intermediate to longer length. The package of 1% solution is often mixed with 10% glucose in equal parts to form a hyperbaric spinal anesthetic that is used for perineal and abdominal surgery. With tetracaine, TNS occurs at a lower rate than with lidocaine spinal anesthesia. The addition of phenylephrine may play a role in the development of TNS.[71-73] Mepivacaine is very similar to lidocaine and has been used since the 1960s for spinal anesthesia. The incidence of TNS reported after mepivacaine spinal anesthesia varies widely, with rates from 0% to 30%.[74-76] Ropivacaine was introduced in 1996. For applications in spinal anesthesia, ropivacaine has been found to be less potent than spinal bupivacaine. Ropivacaine has significantly less risk of TNS than spinal lidocaine. Studies comparing ropivacaine to bupivacaine in spinal anesthesia are in progress.[77-79] Table 4 shows some of the local anesthetics used for spinal anesthesia and dosage duration, and concentration for different levels of spinal blockade.[79-88]
Additives to Local Anesthesia Vasoconstrictors have been added to local anesthetics, and both epinephrine and phenylephrine have been studied. Anesthesia is intensified and prolonged with smaller doses of local anesthetics when epinephrine or phenylephrine is added. Tissue vasoconstriction is produced, thus limiting the systemic reabsorption of the local anesthetic and prolonging the duration of action by keeping the local anesthetic in contact with the nerve fibers. However, complications can occur after the use of vasoconstrictors in spinal anesthesia. In some studies, epinephrine was implicated as the cause of cauda equina syndrome because of anterior spinal artery ischemia, but most studies do not demonstrate an association between the use of vasoconstrictors for spinal anesthesia and the incidence of cauda equina.[89,90] Phenylephrine has been shown to be associated with TNS.[73,91] Epinephrine Epinephrine is thought to work by decreasing local anesthetic uptake and thus prolonging the spinal blockade of some local anesthetics. Vasoconstrictors can cause ischemia, and there is concern of spinal cord ischemia when epinephrine is added to spinal anesthetics. Animal models have not shown any decrease in spinal cord blood flow or increase in spinal cord ischemia when epinephrine is given for spinal blockade, even though some neurologic complications associated with the addition of epinephrine exist.[47,49,92,93] Epinephrine comes packaged as 1mg in 1 mL, which is a 1:1000 solution. The dosage of epinephrine added to local anesthetics is 0.1 to 0.5mg, meaning 0.1mLto 0.5mLis added to the local anesthetic solution. Adding 0.1mLof epinephrine to 10 mL of local anesthetic yields a 1:100,000 concentration of epinephrine. Adding 0.1 mL of epinephrine to 20 mL of local anesthetic yields a 1:200,000 concentration, and so on (0.1 mL in 30 mL = 1:300,000). Calculation of epinephrine concentration does not need to be complex if this simple formula is remembered. Epinephrine prolongs the duration of spinal anesthesia.[94-96] In the past, it was thought that epinephrine had no effect on hyperbaric spinal bupivacaine using two-segment regression to test neural blockade.[97] However, a recent study showed that epinephrine prolongs the duration of hyperbaric spinal bupivacaine when pinprick, transcutaneous electrical nerve stimulation (TENS) equivalent to surgical stimulation (at umbilicus, pubis, knee, and ankle), and tolerance of a pneumatic thigh tourniquet were used to determine neural blockade.[98] Currently there is controversy regarding prolongation of spinal bupivacaine neural blockade when epinephrine is added.[99-102] The same controversy exists about the prolongation of spinal lidocaine with epinephrine.[103-107] Epinephrine likely prolongs spinal blockade with both bupivacaine and lidocaine. All three types of opioid receptors are found in the dorsal horn of the spinal cord and serve as the target for intrathecal opioid injection. Receptors are located on spinal cord neurons and terminals of afferents originating in the dorsal root ganglion. Fentanyl, sufentanil, meperidine, and morphine have all been used intrathecally. Side effects that may be seen include pruritus, nausea and vomiting, and respiratory depression.[108-112] Alpha-2-adrenergic agonists can be added to spinal injections of local anesthetics in order to enhance pain relief and prolong sensory block and motor block. Enhanced postoperative analgesia has been demonstrated in cesarean deliveries, fixation of femoral fractures, and knee arthroscopies when clonidine was added to the local anesthetic solution. Clonidine prolongs the sensory and motor blockade of a local anesthetic after spinal injection.[113-115] Sensory blockade is thought to be mediated by both presynaptic and postsynaptic mechanisms. Clonidine induces hyperpolarization at the ventral horn of the spinal cord and facilitates the action of the local anesthetic, thus prolonging motor blockade when used as an additive. However, when used alone in intrathecal injections, clonidine does not cause motor block or weakness.116 Side effects can occur with the use of spinal clonidine, and include hypotension, bradycardia, and sedation. Currently, neuraxial clonidine is approved by the Food and Drug Administration (FDA) for intractable neuropathic pain.[117,118] Acetylcholinesterase inhibitors prevent the breakdown of acetylcholine and produce analgesia when injected intrathecally. The antinociceptive effects are due to increased acetylcholine and generation of nitricoxide. It has been shown in a rat model that diabetic neuropathy can be alleviated after intrathecal neostigmine injection.[119] Side effects of intrathecal neostigmine include nausea and vomiting, bradycardia requiring atropine, anxiety, agitation, restlessness, and lower extremity weakness.[120-122] Although spinal neostigmine provides extended pain control, the side effects that occur do not allow its widespread use. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 02/20/2016(+ 2016 Dates) | |
| 01/27/2016 | |
| 03/17/2016 | |
| 04/20/2016 | |
| 09/23/2016 | |
| 10/01/2024 |
![[advertisement] gehealthcare](../../../files/banners/banner1_250x600/GEtouch(250X600).gif)

![[advertisement] concertmedical](../../../files/bk-nysora-ad.jpg)




















Post your comment