Caudal Anesthesia
Caudal anesthesia was first described at the turn of last century by two French physicians, Fernand Cathelin and Jean-Anthanase Sicard. The technique predated the lumbar approach to epidural block by several years.
By Kenneth D. Candido, MD and Alon Winnie, MD
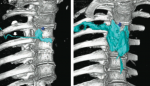
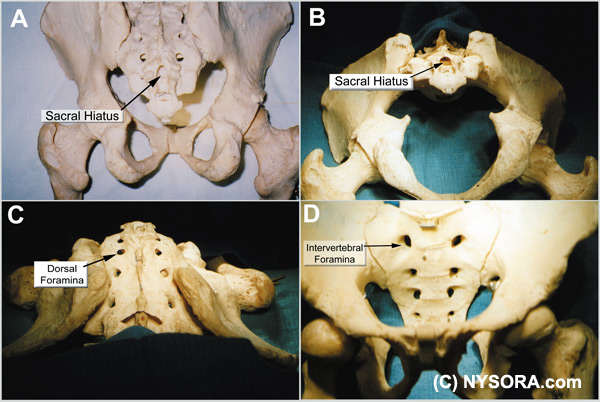 Figure 1. A: Skeletal model demonstrating the sacral hiatus and its relationship to the coccyx and sacrum. The fifth inferior articular processes project caudally and flank the sacral hiatus as sacral cornuae. B: Skeletal specimen viewed from inferior to the sacral hiatus. The hiatus is seen as the oval shaped opening at the 12 o' clock position in the photograph. C: Skeletal specimen of the sacrum viewed from craniad to caudad demonstrating the five dorsal foramina, situated bilaterally. D: Skeletal specimen of the sacrum demonstrating the ventral sacral surface. Note the five bilateral intervertebral foramina, paired on either side of the midline, defined by the retention screws used to hold the specimen together. Anatomic Considerations The sacrum is a large triangularly shaped bone formed by the fusion of the five sacral vertebrae. It has a blunted, caudal apex that articulates with the coccyx. Its superior, wide base articulates with the fifth lumbar vertebra at the lumbosacral angle (Figure 1 A-D). Its dorsal surface is convex and has a raised interrupted median crest with four (sometimes three) spinous tubercles representing fused sacral spines. Flanking the median crest, the posterior surface is formedby fused laminae. Lateral to the median crest, four pairs of dorsal foramina lead into the sacral canal through intervertebral foraminae, each of which transmits the dorsal ramus of a sacral spinal nerve (see Figure 1B). Below the fourth (or third) spinous tubercle an arched sacral hiatus is identified in the posterior wall of the sacral canal, due to the failure of the fifth pair of laminae tomeet, exposing the dorsal surface of the fifth sacral vertebral body. The caudal opening of the canal is the sacral hiatus (see Figure 1A and B), roofed by the firm elastic membrane, the sacrococcygeal ligament, which is an extension of the ligamentum flavum. The fifth inferior articular processes project caudally and flank the sacral hiatus as sacral cornuae, connected to the coccygeal cornua by intercornual ligaments. The sacral canal is formedby the sacral vertebral foramina and is triangular in shape. It is a continuation of the lumbar spinal canal. Each lateral wall presents four intervertebral foramina, through which the canal is contiguous with the pelvic and dorsal sacral foramina. The posterior sacral foramina are smaller than their anterior counterparts. The sacral canal contains the cauda equina (including the filum terminale) and the spinal meninges. Near its midlevel (typically the middle one third of S2, but varying from the midpoint of S1 to the midpoint of S3) the subarachnoid and subdural spaces cease to exist, and the lower sacral spinal roots and filum terminale pierce the arachnoid and dura maters.[3,4] The lowest margin of the filum terminale emerges at the sacral hiatus and traverses the dorsal surface of the fifth sacral vertebra and the sacrococcygeal joint to reach the coccyx. The fifth spinal nerves also emerge through the hiatus medial to the sacral cornua. The sacral canal contains the epidural venous plexus, which generally terminates at S4, but which may continue more caudally. Most of these vessels are concentrated in the anteriolateral portion of the canal. The remainder of the sacral canal is filled with adipose tissue, which is subject to an age-related decrease in its density. This change may be responsible for the transition from the predictable spread of local anesthetics administered for caudal anesthesia in children to the limited and unpredictable segmental spread seen in adults.[5]
Considerable variability occurs in sacral hiatus anatomy among individuals of seemingly similar backgrounds, race, and stature.[1] As individuals age, the overlying ligaments and the cornua thicken significantly. The hiatal margins often defy recognition by even skilled fingertips. The practical problems related to caudal anesthesia are mainly attributable to wide anatomic variations in size, shape, and orientation of the sacrum. Trotter[3] summarized the major anatomic variations of the sacrum. The sacral hiatus may be almost closed, asymmetrically open, or widely open secondary to anomalies in the pattern of fusion of the laminae of the sacral arches. Sacral spina bifida was noted in about 2% of males, and in 0.3% of females. The anteroposterior depth of the sacral canal may vary from less than 2 mm to greater than 1 cm. Individuals with sacral canals having anteroposterior diameters less than about 3 mm may not be able to accommodate anything larger than a 21-gauge needle (5% of the population).[1] Additionally, the lateral width of the sacral canal varies significantly. Since the depth and width of the canal may vary, the volume of the canal itself may also vary. Trotter found that sacral volumes varied between 12 and 65 mL, with a mean volume of 33 mL.[3] A magnetic resonance imaging (MRI) study in 37 adult patients found the volume (excluding the foramina and dural sac) to be 14.4 mL, with a range of 9.5 to 26.6 mL.[6] Patients with smaller capacities may not be able to accommodate the typical volumes of local anesthetics administered for epidural anesthesia via the caudal route. In a cadaver study of 53 specimens, the mean distance between the tip of the dural sac and the upper edge of the sacral hiatus as denoted by the sacrococcygeal membrane was 45 mm, with a range of 16 to 75 mm.[3] In the MRI study mentioned earlier, the mean distance was found to be 60.5 mm, with a range of 34 to 80 mm.[6] The sacrococcygeal membrane could not be identified in 10.8% of subjects using MRI.[6] A recent anatomic evaluation of 92 isolated sacra found that 42% of cases had both a hiatus and cornu; 4% of the cases showed an absent hiatus. The apex of the sacral hiatus, in that study, was noted in 64% of cases to exist at the S4 level. The hiatus was closed in 3% of cases.[7] The sacral foramina afford anatomic passages that permit the spread of injected solutions such as local anesthetics and adjuvants (see Figures 1C and D). The posterior sacral foramina are essentially sealed by the multifidus and sacrospinalis muscles, but the anterior foramina are unobstructed by muscles and ligaments, permitting ready egress of solutions through them.[8] The sacral curvature also varies substantially.[9] This variability tends to be more pronounced in males than in females. The clinical significance of this finding is that a noncurving epidural needle will more likely pass easily into the canal of females than males. The angle between the axis of the lumbar canal and the sacral canal varies between 7 and 70 degrees in subjects with marked lordosis. The clinical implication of this finding is that the cephalad flow of caudally injected solutions may be more limited in lordotic patients with exaggerated lumbosacral angles than in those with flatter lumbosacral angles, in whom the axes of the lumbar and sacral canals are more closely aligned. Indications for Caudal Epidural Block The indications for caudal epidural block are essentially the same as those for lumbar epidural block, but its use may be preferred when sacral nerve spread of anesthetics and adjuvants is preferred over lumbar nerve spread. The unpredictability of ascertaining consistent cephalad spread of anesthetics administered through the caudal canal limits the usefulness of this technique when it is essential to provide lower thoracic and upper abdominal neuraxial blockade. Though this modality is described for perioperative use (diminishing role) and for managing chronic pain in adults (increasing role), it is essential to recognize that caudal block has an extremelywide range of applicability (Table 1).[10-13]
Other newer indications in adults bear special mention and will be described later, including the performance of percutaneous epidural neuroplasty;[14,15] the use of caudal analgesia following lumbar spinal surgery;[16] caudal analgesia after emergency orthopedic lower extremity surgery;[17] administering local anesthetic adjuvants for postoperative analgesia;[18] and caudal block for performing neurolysis for intractable cancer pain.[19] The Technique Of Caudal Epidural Block The technique of caudal epidural block involves palpation, identification and puncture.[1] Patients are evaluated as for any epidural block, and the indications and relative and absolute contraindications to its performance are identical. A full complement of noninvasive monitors is applied, and baseline vital signs are assessed. One must decide whether a continuous or single-shot technique will be employed. For continuous techniques, a Tuohy-type needle with a lateral facing orifice is preferred. Patient Positioning Several positions can be used in adults, compared with the lateral decubitus position in neonates and children. The lateral position is efficacious in pediatrics because it permits easy access to the airway when general anesthesia or heavy sedation has been administered prior to performing the block. In pediatric patients, blocks may be performed with the patient fully anesthetized; the same is not recommended for older children and adults. In adults, the prone position is the most frequently utilized, but the lateral decubitus position or the knee-chest (also known as knee-elbow) position may be employed. In the prone position, the procedure table or operating room table should be flexed, or a pillow may be placed beneath the symphysis pubis and iliac crests to produce slight flexion of the hips. This maneuver makes palpation of the caudal canal easier. The legs are separated with the heels rotated outward to smooth out the upper part of the anal cleft while relaxing the gluteal muscles. For placement of caudal epidural block in the parturient, the woman is in the lateral (Sim position) or in the knee-elbow position. 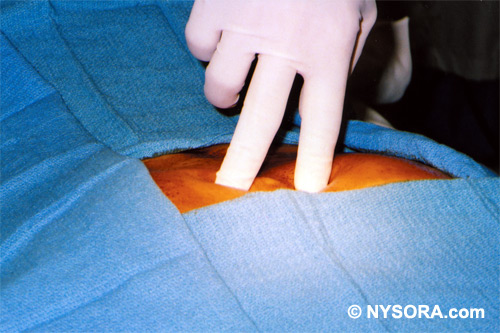 Figure 2. Technique of palpating the midline over the sacral hiatus. The index and middle fingers of the palpating fingers are spread over the fifth sacral vertebral body. The sacrococcygeal ligament lies directly beneath the palpating fingers. Anatomic Landmarks A dry gauze swab is placed in the anal cleft to protect the anal area and genitalia from povidone-iodine (Betadine) or other disinfectants (especially alcohol) used to sterilize the skin. The skin folds of the buttocks are useful guides in locating the underlying sacral hiatus. Alternatively, a triangle may be marked on the skin over the sacrum, using the posterior superior iliac spines (PSIS) as the base, with the apex pointing inferiorly (caudally). Normally, this apex sits over or immediately adjacent to the sacral hiatus. The hiatus is marked and the tip of the index finger is placed on the tip of the coccyx in the natal cleft while the thumb of the same hand palpates the two sacral cornua located 3-4 cm more rostrally at the upper end of the natal cleft. The sacral cornua may be identified by gently moving the palpating index finger from side to side (Figure 2). The palpating thumb should sink into the hollow between the two cornua, as if between two knuckles of a fist.[1] A sterile skin preparation and draping of the entire region is performed in the usual fashion.
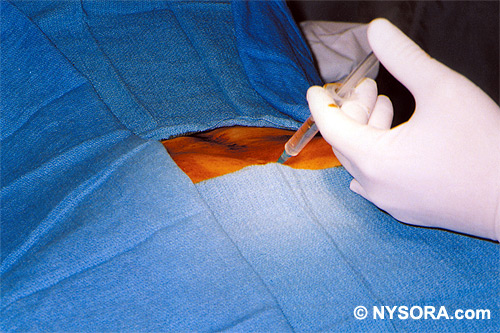
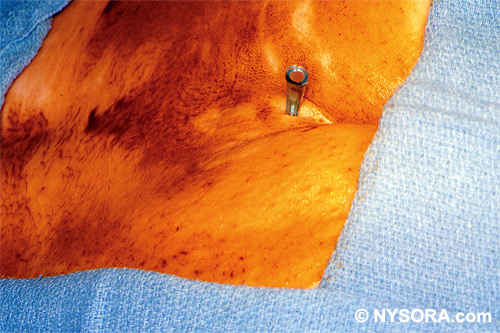
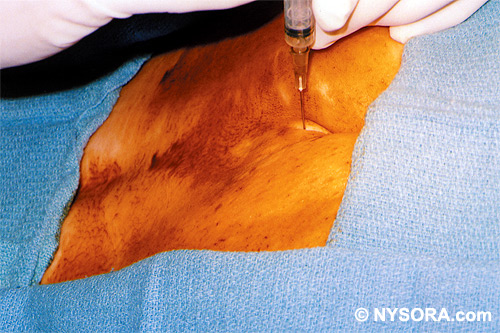
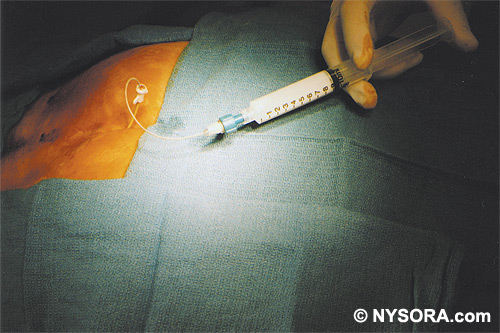
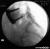
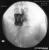
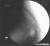
Technique A small-gauge 1.5-in. needle is then utilized to infiltrate the skin over the sacral hiatus using 3-5 mL of 1-1.5% plain lidocaine HCl (Figures 3 through 5). If fluoroscopy is utilized, a lateral view is obtained to demonstrate the anatomic boundaries of the sacral canal. We routinely leave the local anesthetic infiltration needle in situ for this view, since it demonstrates whether the approach is at the appropriate level for subsequent advancement of the epidural needle.With fluoroscopy, the caudal canal appears as a translucent layer posterior to the sacral segments (Figure 6). The median sacral crest is visualized as an opaque line posterior to the caudal canal. The sacral hiatus is usually visualized as a translucent opening at the base of the caudal canal. The coccyx may be seen articulating with the inferior surface of the sacrum. Once the tissues overlying the hiatus have been anesthetized, a 17- or 18-gauge Tuohy-type needle is inserted either in the midline or, using a lateral approach, into the caudal canal (Figures 7 and 8). A feeling of a slight "snap" may be appreciated when the advancing needle pierces the sacrococcygeal ligament. Once the needle reaches the ventral wall of the sacral canal, it is slowly withdrawn and reoriented, directing it more cranially (by depressing the hub and advancing) for further insertion into the canal (Figure 9). We utilize the anteroposterior view once the epidural needle is safely situated within the confines of the canal, and the epidural catheter is advanced cephalad. In this projection, the intermediate sacral crests appear as opaque vertical lines on either side of the midline. The sacral foramina are visualized as translucent and nearly circular areas lateral to the intermediate sacral crests. The presence of intestinal gas may obfuscate the recognition of these structures. A syringe loaded with either air or saline containing a small air bubble is attached to the needle, and the loss-of-resistance technique is used to establish entry into the epidural space. 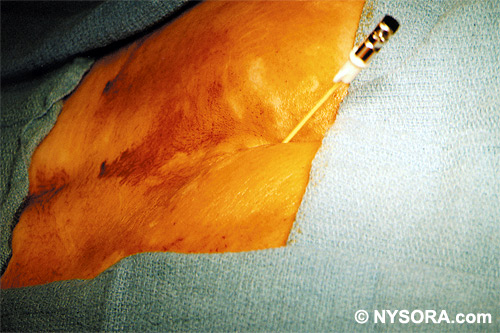 Figure 7. The 17-gauge needle has been advanced from the skin into the sacral hiatus through the sacrococcygeal ligament. Usually, when fluoroscopy is not available to verify correct needle placement, a syringe loaded with air or saline is attached to the needle and the loss-of-resistance technique is employed to identify the epidural space, as for conventional lumbar or cervical epidural injections. 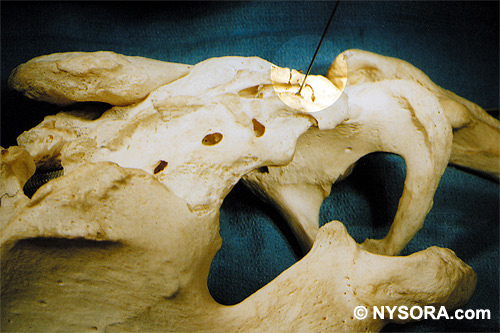 Figure 8. Skeletal specimen demonstrating the needle introducer from the 17-gauge needle situated correctly in the caudal epidural space, traversing the sacrococcygeal ligament (removed) and entering the sacral hiatus (lateral view). 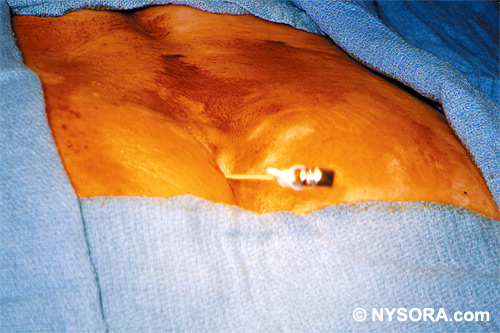 Figure 9. Caudocranial view of the 17-gauge needle situated correctly through the sacrococcygeal ligament into the sacral hiatus.
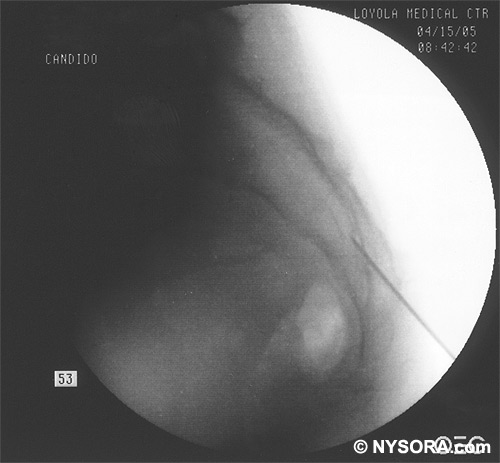
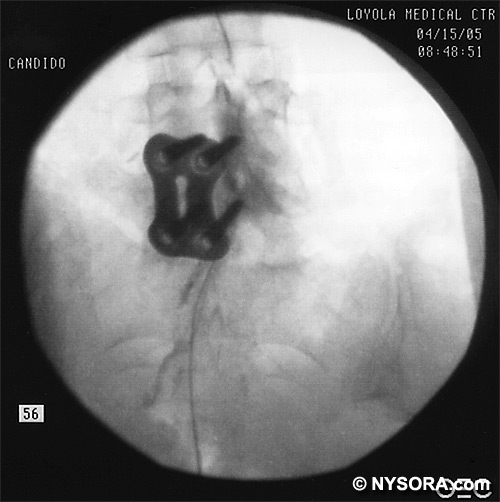
A Whoosh test has been described for identifying correct needle placement in the caudal canal. This characteristic sound has been noted during auscultation of the thoracolumbar region during the injection of 2 to 3 mL of air into the caudal epidural space.[20] The test has been modified in pediatrics, wherein local anesthetic, and not air injection, is auscultated during the performance of the block. Of the 108 patients with a successful block in one study, 98 had a positive test, with no false-positive results.[21] Once the correct placement of the needle is confirmed, a catheter is inserted to the desired location (depth) (Figure 10), and its position confirmed fluoroscopically when desired (Figures 11 and 12). 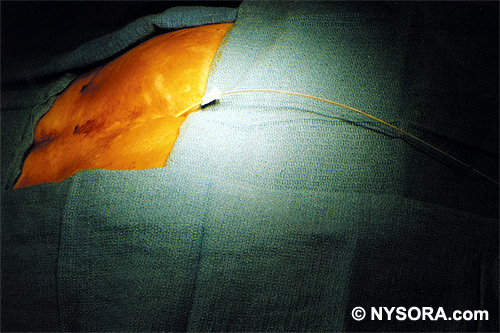 Figure 10. A continuous catheter with a stylet in place is shown. The catheter is advanced through either the short over the needle catheter that was left in situ (shown), or through a 17 to 18-gauge steel needle placed in the canal. 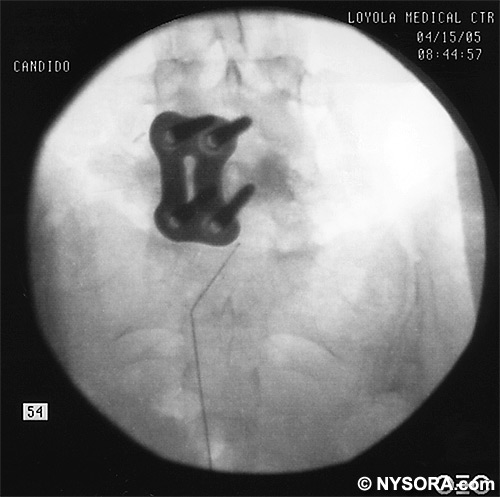 Figure 11. Anteroposterior fluoroscopic image depicting proper placement of the needle. The patient's hardware from previous fusion surgery is also seen. 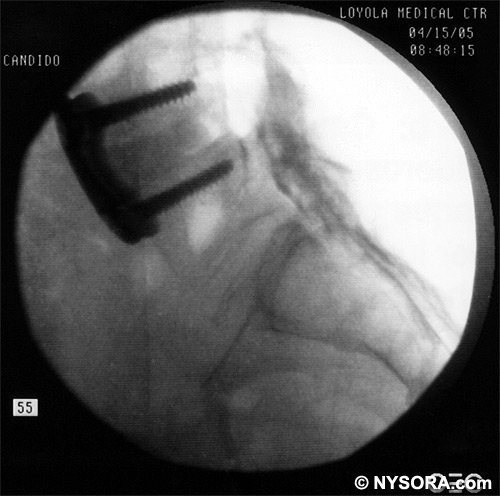 Figure 12. Lateral fluoroscopic image depicting radiopaque contrastmediumin the caudal and lower lumbar epidural spaces. The image shows considerable spread, both anteriorly and posteriorly, following the injection of 2 mL of dye.
Injected solutions may be absorbed very rapidly by bone marrow and toxic drug reactions result. In this situation, pain is typically noted over the caudal part of the sacrum during the injection. If this occurs, the needle should be withdrawn slightly and rotated on its axis until it can be reinserted in a slightly different direction.[23-25] If injection is made anterior to the sacrum (between the sacrum and coccyx), it is possible to perforate the rectum, or, in parturients, the baby's head may be injured. This limits the use of caudal block in laboring women once the presenting part has descended into the perineum. Inadvertent venous puncture also may occur, and the incidence of this has been reported to be about 0.6%.[26]
Caudal block may be used with a single-shot or continuous catheter technique. For continuous block, the catheter may be advanced anterogradely (conventionally) or retrogradely. Continuous caudal block may be performed in retrograde fashion using needle insertion into the lumbar epidural space, but directed inferiorly instead of superiorly. In one study of 10 patients, epidural catheters were advanced through 18-gauge Tuohy-type epidural needles in retrograde fashion from the L4-5 interspace. This technique was associated with a 20% failure rate with the catheter going into the paravertebral or retrorectal spaces, despite easy epidural space entry.[27] Using the conventional approach, a Huber-tipped Tuohy needle is used as a conduit to pass the epidural catheter into the canal. This needle has a ski-like tip that limits its being caught or snagged on the sacral periosteum. The needle is inserted with its shoulder facing anteriorly and its orifice dorsally. Alternatively, a standard 16- or 17-gauge catheter-over-needle assemblage (angiocatheter) may serve as the introducing needle for subsequent catheter placement. The catheter is advanced with fluoroscopic guidance, especially when it is performed for chronic pain management in failed back surgery syndrome. The catheters should be advanced gently, since there have been reports of dural puncture with rapid or aggressive advancement. The lateral and anteroposterior views should be obtained to demonstrate placement of the catheter in the epidural space (lateral view, see Figure 6) and to follow its path in a cephalad or cephalolateral direction (anteroposterior view, see Figure 11).When the desired level is attained, iodinated non-ionic contrast media may be injected, followed by the injection of local anesthetics, corticosteroids or adjuncts (Figures 13 and 14).We usually do not advance the catheter higher than the level of the L4 vertebral body, although we have occasionally advanced it to the L1 or L2 level. Some authorities suggest avoiding advancement more than 8-12 cm cephaladly.
Characteristics & Indications Of Caudal Epidural Block In Adults Characteristics of the Blockade Caudal epidural block results in sensory and motor block of the sacral roots and limited autonomic block. The sacral contribution of the parasympathetic nervous system is blocked, causing loss of visceromotor function of the bladder and intestines distal to the colonic splenic flexure. Sympathetic block, though limited compared with lumbar or thoracic epidural block, does occur. However, the sympathetic outflow from the spinal cord ends at the L2 level, and, therefore, caudal block should not routinely result in peripheral vasodilatation of the lower extremities to the degree witnessed with lumbar epidural blockade. Caudal epidural local anesthetic block in adults may be chosen for surgeries of the lower abdomen, perineum, or lower extremities. The local anesthetic mixtures and doses are similar to those for lumbar epidural block (Table 2).
Spread of the Local Anesthetic Solutions The large capacity of the sacral canal accommodates correspondingly large volumes of solution; significant volumes may be lost through the wide anterior sacral foramina. Therefore, the caudal dose requirements of local anesthetics are significantly larger to effect the same segmental spread than are the corresponding lumbar doses. Roughly twice the lumbar epidural local anesthetic dose is needed for caudal blockade to attain similar levels of analgesia and anesthesia, and solutions injected in the caudal space take longer to spread (see Table 2). Bromage noted that age is not correlated with caudal segmental spread in adults and the upper level of analgesia resulting from 20-mL doses of local anesthetic solution varies widely between S2 and T8.[1] This unpredictability limits the usefulness of applying caudal anesthesia for surgical procedures that require cephalad analgesia levels above the pelvic level or the umbilicus. A recent study reconfirmed Bromage's findings. In 172 women undergoing minor gynecologic surgery using caudal anesthesia with 20 mL of 1.5% lidocaine, the highest sensory dermatome level reached was below T10.[28]
Indications in Adults Caudal block is indicated whenever the area of surgery involves the sacral and lower lumbar nerve roots. The technique is suitable for anal surgery (hemorrhoidectomy and anal dilatation), gynecologic procedures, surgery on the penis or scrotum, and lower limb surgeries. Using a catheter technique, it is possible to use caudal epidural block for vaginal hysterectomy and inguinal herniorrhaphy. Caudal epidural block is used less frequently than lumbar or even thoracic epidural block for providing perioperative analgesia in adults. The pelvis enlarges markedly in puberty while the epidural fat in the lumbosacral region undergoes compaction and increased fibrous content. This hinders cephalad spread of solutions particularly when compared with the spread in children. As an alternative to caudal epidural block in adults, one might consider a median approach to transsacral epidural block. In the original description of that technique, 87% of blocks were successful for transurethral resection of bladder tumors, vs 100% success for sacral procedures. Anesthesia level, side effects, and hemodynamics were similar between the two groups studied in that initial report.[29] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 02/20/2016(+ 2016 Dates) | |
| 01/27/2016 | |
| 03/17/2016 | |
| 04/20/2016 | |
| 09/23/2016 | |
| 10/01/2024 |
![[advertisement] gehealthcare](../../../files/banners/banner1_250x600/GEtouch(250X600).gif)

![[advertisement] concertmedical](../../../files/bk-nysora-ad.jpg)















Post your comment