Spinal and Epidural Block
|
Authors: Wing Hong Kwok and Manoj Karmakar Introduction Ultrasound scanning (US) can offer several advantages when used to guide placement of the needle for centroneuraxial blocks (CNBs). It is noninvasive, safe, simple to use, can be performed expeditiously, provides real-time images, is devoid from adverse effects, and it may be beneficial in patients with abnormal or variant spinal anatomy. When used for chronic pain interventions, US also eliminates or reduces exposure to radiation. In expert hands, the use of US for epidural needle insertion was shown to reduce the number of puncture attempts, (1-4) improve the success rate of epidural access on the first attempt, (2) reduce the need to puncture multiple levels, (2-4) and improve patient comfort during the procedure. (3) These advantages led the National Institute of Clinical Excellence (NICE) in the United Kingdom to recommend the routine use of ultrasound for epidural blocks. (5) Incorporating these recommendations into clinical practice, however, has met significant obstacles. As one example, a recent survey of anesthesiologists in the United Kingdom showed that >90% of respondents were not trained in the use of US to image the epidural space. (6) In this chapter, we describe techniques of US imaging of the spine, the relevant sonoanatomy, and practical considerations for using US-guided CNB and nerve blocks close to the centroneuroaxis. Historical Background Bogin and Stulin were probably the first to report using US for central neuraxial interventional procedures. (7) In 1971, they described using US to perform lumbar puncture. (7) Porter and colleagues, in 1978, used US to image the lumbar spine and measure the diameter of the spinal canal in diagnostic radiology. (8) Cork and colleagues were the first group of anesthesiologists to use US to locate the landmarks relevant for epidural anesthesia. (9) Thereafter, US was used mostly to preview the spinal anatomy and measure the distances from the skin to the lamina and epidural space before epidural puncture. (10,11) More recently, Grau and coworkers, from Heidelberg in Germany, conducted a series of studies, significantly contributing to the current understanding of spinal sonography. (1-4,12-15) These investigators described a two-operator technique consisting of real-time US visualization of neuraxial space using a paramedian sagittal axis and insertion of the needle through the midline to accomplish a combined spinal-epidural block. (4) The quality of the US image at the time, however, was substantially inferior to that of today's equipment, thus hindering acceptance and further research in this area. Recent improvements in US technology and image clarity have allowed for much greater clarity during imaging of the spine and neuraxial structures. (16,17) Ultrasound Imaging of the Spine Basic Considerations Because the spine is located at a depth, US imaging of the spine typically requires the use of low-frequency ultrasound (5-2 MHz) and curved array transducers. Low-frequency US provides good penetration but unfortunately, it lacks the spatial resolution at the depth (5-7 cm) at which the neuraxial structures are located. The osseous framework of the spine, which envelops the neuraxial structures, reflects much of the incident US signal before it reaches the spinal canal, presenting additional challenges in obtaining good quality images. Recent improvements in US technology, the greater image processing capabilities of US machines, the availability of compound imaging, and the development of new scanning protocols have improved the ability to image the neuraxial space significantly. As a result, today it is possible to reasonably accurately delineate the neuraxial anatomy relevant for CNB. Also of note is that technology once only available in the high-end, cart-based U.S. systems is now available in portable US devices, making them even more practical for spinal sonography and US-guided CNB applications. Ultrasound Scan Planes
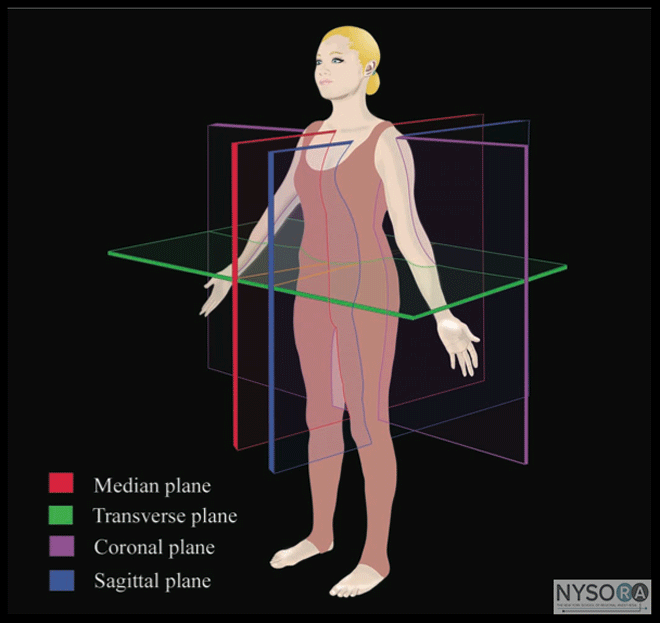
Figure 1: Anatomic planes of the body 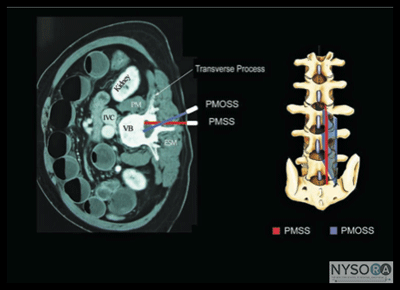
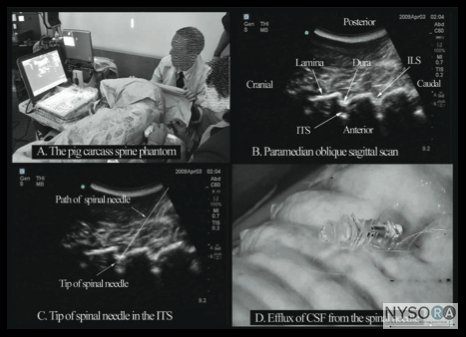
Figure 2: Paramedian sagittal scan (PMSS) of the lumbar spine. The PMSS is represented by the red color and the paramedian oblique sagittal axis of scan (PMOSS) is represented by the blue color. Note how the plane of imaging during a PMOSS is tilted slightly medially. This is done to ensure that most of the ultrasound energy enters the spinal canal through the widest part of the interlaminar space. ICV, inferior vena cava; VB, vertebral body; PM, psoas muscle; ESM, erector spinae muscle. Although anatomic planes have already been described else- where in this text, the importance of understanding them for imaging of the neuraxial space dictates another, more detailed review. There are three anatomical planes: median, transverse, and coronal (Figure 1). The median plane is a longitudinal plane that passes through the midline bisecting the body into equal right and left halves.The sagittal plane is a longitudinal plane that is parallel to the median plane and perpendicular to the ground. Therefore, the median plane also can be defined as the sagittal plane that is exactly in the middle of the body (median sagittal plane). The transverse plane, also known as the axial or horizontal plane, is parallel to the ground. The coronal plane, also known as the frontal plane, is perpendicular to the ground. A US scan of the spine can be performed in the transverse (transverse scan) or longitudinal (sagittal scan) axis wthe patient in the sitting, lateral decubitus, or prone position. The two scanning planes complement each other during a US examination of the spine. A sagittal scan can be performed through the midline (median sagittal scan) or through a paramedian (paramedian sagittal scan) plane. Grau et al suggested a paramedian sagittal plane to visualize the neuraxial structures.(12) The US visibility of neuraxial structures can be further improved when the spine is imaged in the paramedian oblique sagittal. During a paramedian oblique sagittal scan (PMOSS), the transducer is positioned 2 to 3 cm lateral to the midline (paramedian) in the sagittal axis and it is also tilted slightly medially, that is, toward the midline (Figure 2). The purpose of the medial tilt is to ensure that the US signal enters the spinal canal through the widest part of the interlaminar space and not the lateral sulcus of the canal. Sonoanatomy of the Spine Detailed knowledge of the vertebral anatomy is essential to understand the sonoanatomy of the spine. Unfortunately, cross-sectional anatomy texts describe the anatomy of the spine in traditional orthogonal planes, that is, the transverse, sagittal, and coronal planes. This often results in difficulty interpreting the spinal sonoanatomy because US imaging is generally performed in an arbitrary or intermediary plane by tilting, sliding, and rotating the transducer. Moreover, currently there are limited data on spinal sonography or on how to interpret US images of the spine. Several anatomic models recently became available that can be used to learn musculoskeletal US imaging techniques (human volunteers), the sonoanatomy relevant for peripheral nerve blocks (human volunteers or cadavers), and the required interventional skills (tissue mimicking phantoms, fresh cadavers). However, few models or tools are available to learn and practice spinal sonoanatomy or the interventional skills required for US-guided CNB. Karmakar and colleagues recently described the use of a "water-based spine phantom" (Figure 3A) to study the osseous anatomy of the lumbosacral spine (16,18) and a "pig carcass phantom" model (19) (Figure 4A) to practice the hand-eye coordination skills required to perform US-guided CNB. (19) Computer-generated anatomic reconstruction from the Visible Human Project data set that corresponds to the US scan planes is another useful way of studying the sonoanatomy of the spine. Multiplanar three-dimensional (3D) reconstruction from a high-resolution 3D computed tomography (CT) data set of the spine can be used to study and validate the sonographic appearance of the various osseous elements of the spine (Figure 5). Water-Based Spine Phantom 
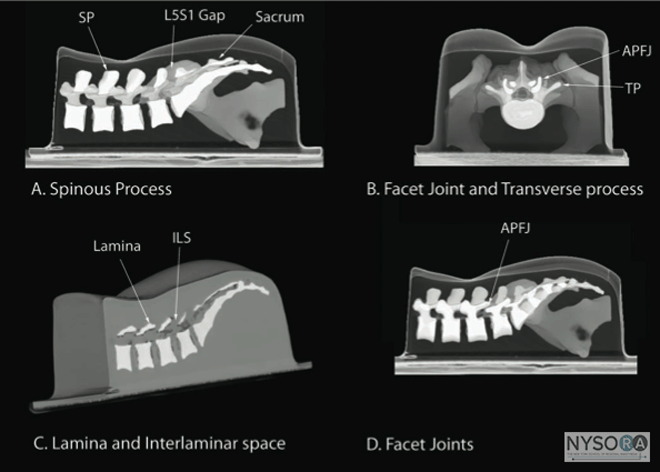
Figure 3: (A) The water-based spine phantom and sonograms of the spinous process in the (B) transverse and (C) midsagittal or median axes, and (D) a scan through the interspinous space. SP, spinous process; ISS, interspinous space; TP, transverse process; AP, articular process; SC, spinal canal; VB, vertebral body; TS, transverse scan; SS, sagittal scan. The water-based spine phantom (18) is based on a model described previously by Greher and colleagues to study the osseous anatomy of relevance to US-guided lumbar facet nerve block. (20) The model is prepared by immersing a commercially available lumbosacral spine model in a water bath. A low-frequency curved array transducer submerged into water is used to scan in the transverse and sagittal axes (Figure 3A). Each osseous element of the spine produces a "characteristic" sonographic pattern. The ability to recognize these sonographic patterns is an important step toward understanding the sonoanatomy of the spine. Representative US images of the spinous process, lamina, articular processes, and the transverse process from the water-based spine phantom are presented in Figures 3 and 6. The advantage of this water-based spine phantom is that water produces an anechoic (black) background against which the hyperechoic reflections from the bone are clearly visualized. The water-based spine phantom allows a see-through real-time visual validation of the sonographic appearance of a given osseous element by performing the scan with a marker (e.g., a needle) in contact with it (Figure 6A). The described model is also inexpensive, easily prepared, requires little time to set up, and can be used repeatedly without deteriorating or decomposing, as animal tissue-based phantoms do.
Ultrasound Imaging of the Lumbar Spine Sagittal Scan The patient is positioned in the sitting, lateral, or prone position with the lumbosacral spine maximally flexed. The transducer is placed 1 to 2 cm lateral to the spinous process (i.e., in the paramedian sagittal plane) at the lower back with its orientation marker directed cranially. A slight tilt medially during the scan is assumed to insonate in a paramedian oblique sagittal plane. First, the sacrum is identified as a flat hyperechoic structure with a large acoustic shadow anteriorly (Figure 7). 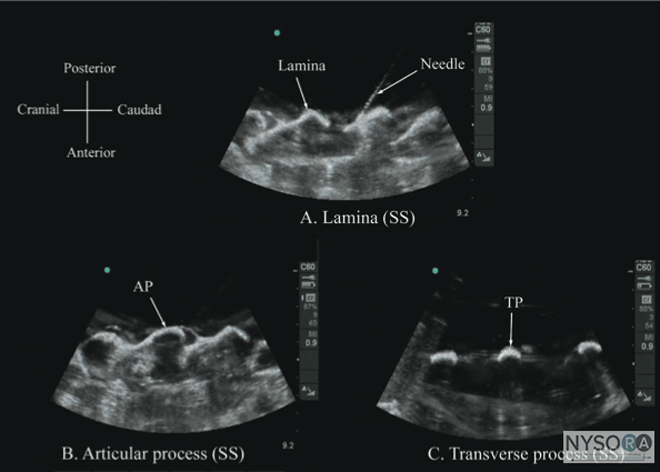
Figure 6: Paramedian sagittal sonogram of the (A) lamina, (B) articular process, and (C) transverse process from the water-based spine phantom. Note the needle in contact with the lamina in (A), a method that was used to validate the sonographic appearance of the osseous elements in the phantom. When the transducer is slid in a cranial direction, a gap is seen between the sacrum and the lamina of the L5 vertebra, which is the L5-S1 interlaminar space, also referred to as the L5-S1gap (Figure 7). (16,17,21) The L3-4 and L4-5 interlaminar spaces can now be located by counting upward (Figure 8). (16,17) The erector spinae muscles are hypoechoic and lie superficial to the laminae. The lamina appears hyperechoic and is the first osseous structure visualized (Figure 8). Because bone impedes the penetration of US, there is an acoustic shadow anterior to each lamina. The sonographic appearance of the lamina produces a pattern that resembles the head and neck of a horse, which Karmakar and colleagues referred to as the "horse head sign" (Figures 5C, 6A, and 8). (16) The interlaminar space is the gap between the adjoining lamina and is the "acoustic window" through which the neuraxial structures are visualized within the spinal canal. The ligamentum flavum appears as a hyperechoic band across adjacent lamina). The posterior dura is the next hyperechoic structure anterior to the ligamentum flavum, and the epidural space is the hypoechoic area (a few millimeters wide) between the ligamentum flavum and the posterior dura. The thecal sac with the cerebrospinal fluid is the anechoic space anterior to the posterior dura (Figure 8). The cauda equina, which is located within the thecal sac, often is seen as multiple horizontal, hyperechoic shadows within the anechoic thecal sac. Pulsations of the cauda equina are identified in some patients. (16,17) The anterior dura also is hyperechoic, but it is not always easy to differentiate it from the posterior longitudinal ligament and the vertebral body because they are of similar echogenicity (isoechoic) and especially closely related. Often, what results is a single, composite, hyperechoic reflection anteriorly that we refer to as the "anterior complex" (Figure 8). (16,17) If the transducer slides medially, that is, to the median sagittal plane, the tips of the spinous processes of the L3-L5 vertebra, which appear as crescent-shaped structures, are seen (Figures 3C, 5A, and 9). (16) The acoustic window between the spinous processes in the median plane is narrow and may prevent clear visualization of the neuraxial structures within the spinal canal. In contrast, if the transducer is moved slightly laterally from the paramedian sagittal plane at the level of the lamina, the articular processes of the vertebra are seen. 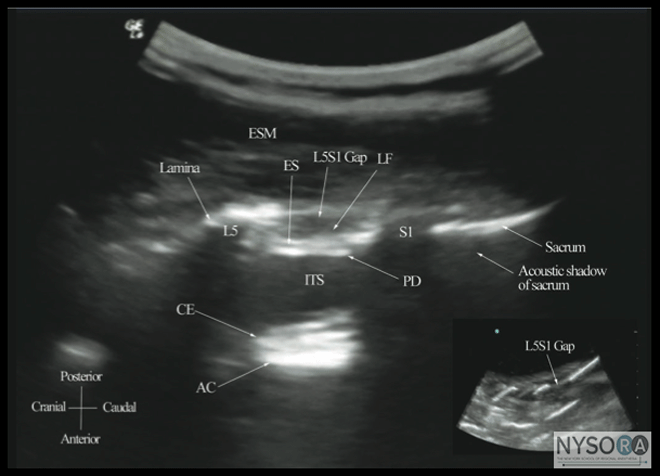
Figure 7: Paramedian sagittal sonogram of the lumbosacral junction. The posterior surface of the sacrum is identified as a flat hyperechoic structure with a large acoustic shadow anterior to it. The dip or gap between the sacrum and the lamina of L5 is the L5-S1 intervertebral space or the L5-S1 gap. ESM, erector spinae muscle; ES, epidural space; LF, ligamentum flavum; PD, posterior dura; ITS, intrathecal space; CE, cauda equina; and AC, anterior complex. The articular processes appear as one continuous, hyperechoic wavy line with no intervening gaps (Figures 5D, 6B, and 10), as seen at the level of the lamina. (16) The articular processes in a sagittal sonogram produce a sonographic pattern that resembles multiple camel humps, which are referred to as the "camel hump sign" (Figures 6B and 10). A sagittal scan lateral to the articular processes brings the transverse processes of the L3-L5 vertebrae into view. The transverse processes are recognized by their crescent-shaped hyperechoic reflections with their concavity facing anteriorly and an acoustic shadow anterior to them (Figures 6C and 11). (22) This produces a sonographic pattern that we refer to as the "trident sign" because of its resemblance to the trident (Latin tridens or tridentis) that is often associated with Poseidon, the god of the sea in Greek mythology, and the Trishula of the Hindu god Shiva (Figure 11). (22) Transverse Scan For a transverse scan of the lumbar spine, the US transducer is positioned over the spinous process (transverse spinous process view) with the patient in the sitting or lateral position. On a transverse sonogram, the spinous process and the lamina on either side are seen as a hyperechoic reflection anterior to which there is a dark acoustic shadow that completely obscures the underlying spinal canal and thus the neuraxial structures (Figures 3B and 12). Therefore, this view is not suitable for imaging of the neuraxial structures but can be useful for identifying the midline when the spinous processes cannot be palpated (e.g., in obese patients). However, if the transducer is slid slid slightly cranially or caudally, it may be possible to perform a transverse scan through the interspinous space (transverse interspinous view) (Figures 3D, 5D, and 13). (16,23) 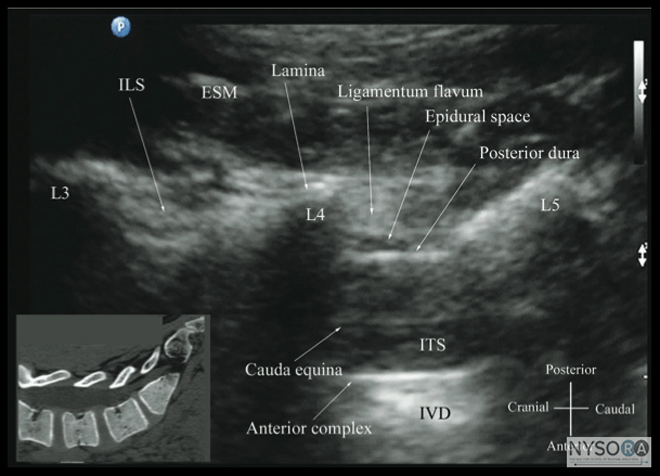
Figure 8: Paramedian oblique sagittal sonogram of the lumbar spine at the level of the lamina showing the L3-4 and L4-5 interlaminar spaces. Note the hypoechoic epidural space (few millimeters wide) between the hyperechoic ligamentum flavum and the posterior dura. The intrathecal space is the anechoic space between the posterior dura and the anterior complex in the sonogram. The cauda equina nerve fibers are also seen as hyperechoic longitudinal structures within the thecal sac. The hyperechoic reflections seen in front of the anterior complex are from the intervertebral disc (IVD). Picture in the inset shows a corresponding computed tomography (CT) scan of the lumbosacral spine in the same anatomic plane as the ultrasound scan. The CT slice was reconstructed from a three-dimensional CT data set from the author’s archive. ESM, erector spinae muscle; ILS, interlaminar space; LF, ligamentum flavum; ES, epidural space; PD, posterior dura; CE, cauda equina; ITS, intrathecal space; AC, anterior complex; IVD, intervertebral disc; L3, lamina of L3 vertebra; L4, lamina of L4 vertebra; L5, lamina of L5 vertebra. It is important to tilt the transducer slightly cranially or caudally to align the US beam to the interspinous space and optimize the US image. In the transverse interspinous view, the posterior dura, thecal sac, and the anterior complex can be visualized (from a posterior to anterior direction) within the spinal canal in the midline and the articular processes, and the transverse processes are visualized laterally (Figure 13). (16,23) The ligamentum flavum is rarely visualized in the transverse interspinous view, possibly due to anisotropy caused by the arch-like attachment of the ligamentum flavum to the lamina. The epidural space is also less frequently visualized in the transverse interspinous scan than in the PMOSS. The transverse interspinous view can be used to examine for rotational deformities of the vertebra, such as in scoliosis. Normally, both the lamina and the articular processes on either side are located symmetrically (Figures 3D, 5D and 13). However, if there is assymmetry, then a rotational deformity of the vertebral column (24) should be suspected and the operator can anitcipate a potentially difficult CNB. Ultrasound Imaging of the Thoracic Spine US imaging of the thoracic spine is more challenging than imaging the lumbar spine; the ability to visualize the neuraxial structures with US may vary with the level at which the imaging is performed. Regardless of the level at which the scan is performed, the thoracic spine is probably best imaged with the patient in the sitting position. In the lower thoracic region (T9-12), the sonographic appearance of the neuraxial structures is comparable to that in the lumbar region because of comparable vertebral anatomy (Figure 14). 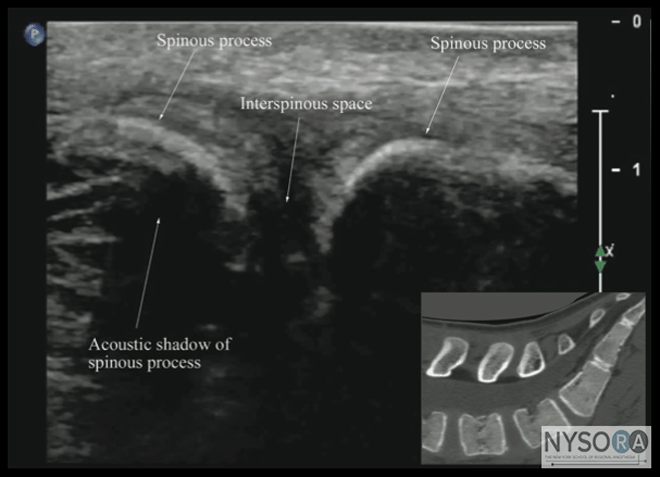
Figure 9: Median sagittal sonogram of the lumbar spine showing the crescent shaped hyperechoic reflections of the spinous processes. Note the narrow interspinous space in the midline. Picture in the inset shows a corresponding computed tomography (CT) scan of the lumbosacral spine through the median plane. The CT slice was reconstructed from a three-dimensional CT data set from the author's archive. However, the acute angulation of the spinous processes and the narrow interspinous and interlaminar spaces in the midthoracic region results in a narrow acoustic window with limited visibility of the underlying neuraxial anatomy (Figure 15). In the only published report describing US imaging of the thoracic spine, Grau and colleagues (13) performed US imaging of the thoracic spine at the T5-6 level in young volunteers and correlated findings with matching magnetic resonance imaging (MRI) images. They reported that the transverse axis produced the best images of the neuraxial structures. Epidural space, however, was best visualized in the paramedian scans. (13) Regardless, US was limited in being able to delineate the epidural space or the spinal cord but was better than MRI in demonstrating the posterior dura. (13) The transverse interspinous view, however, is almost impossible to obtain in the midthoracic region, and therefore the transverse scan provides little useful information for CNB other than to help identify the midline. In contrast, PMOSS, despite the narrow acoustic window, provides more useful information relevant for CNB. The laminae are seen as flat hyperechoic structures with acoustic shadowing anteriorly, and the posterior dura is consistently visualized in the acoustic window (Figures 14 and 15). However, the epidural space, spinal cord, central canal, and the anterior complex are difficult to delineate in the midthoracic region (Figure 15). Ultrasound Imaging of the Sacrum Usually, US imaging of the sacrum is performed to identify the sonoanatomy relevant for a caudal epidural injection. (25) Because the sacrum is a superficial structure, a high-frequency linear array transducer can be used for the scan. (16,25) The patient is positioned in the lateral or prone position with a pillow under the abdomen to flex the lumbosacral spine. 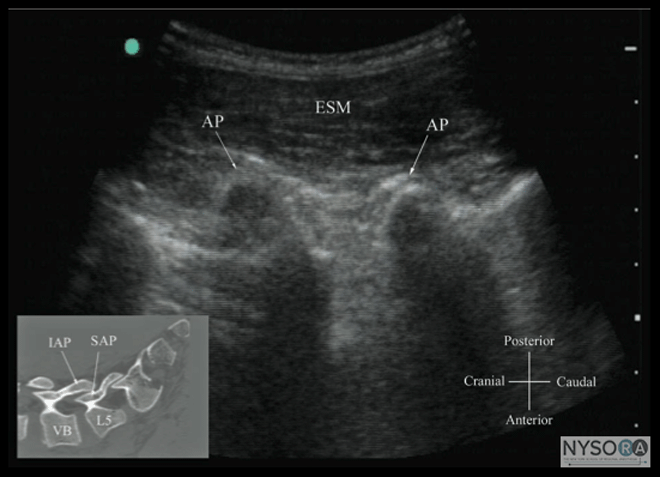
Figure 10: Paramedian sagittal sonogram of the lumbar spine at the level of the articular process (AP) of the vertebra. Note the "camel hump" appearance of the articular processes. Picture in the inset shows a corresponding computed tomography (CT) scan of the lumbosacral spine at the level of the articular processes. The CT slice was reconstructed from a three- dimensional CT data set from the author's archive. ESM, erector spinae muscle; IAP, inferior articular process; SAP, superior articular process. The caudal epidural space is the continuation of the lumbar epidural space and commonly accessed via the sacral hiatus. The sacral hiatus is located at the distal end of the sacrum, and its lateral margins are formed by the two sacral cornua covered by the sacrococcygeal ligament. On a transverse sonogram of the sacrum at the level of the sacral hiatus, the sacral cornua are seen as two hyperechoic reversed U-shaped structures, one on either side of the midline (Figure 16). (16,25) Connecting the two sacral cornua, and deep to the skin and subcutaneous tissue, is a hyperechoic band, the sacrococcygeal ligament. (16,25) Anterior to the sacrococcygeal ligament is another hyperechoic linear structure, which represents the posterior surface of the sacrum. The hypoechoic space between the sacrococcygeal ligament and the bony posterior surface of the sacrum is the caudal epidural space. The two sacral cornua and the posterior surface of the sacrum produce a pattern on the sonogram that we refer to as the "frog eye sign" because of its resemblance to the eyes of a frog (Figure 16). (16) On a sagittal sonogram of the sacrum at the level of the sacral cornua, the sacrococcygeal ligament, the base of sacrum, and the caudal canal are also clearly visualized (Figure 17). (16) Technical Aspects of Ultrasound-Guided Central Neuraxial Blocks "USG CNB can be performed as an off-line or in-line technique. Off-line technique involves performing a prepuncture scan (scout scan) to preview the spinal anatomy, determine the optimal site, depth and trajectory for needle insertion before performing a traditional spinal or epidural injection. (26,27) In contrast, an in-line technique involves performing a real-time USG CNB by a single17 or two (4) operators." Real-time US-guided CNB demands a high degree of manual dexterity and hand-eye coordination. Therefore, the operator should have sound knowledge of the basics of US, be familiar with the sonoanatomy of the spine and scanning techniques, and have the necessary interventional skills before attempting a real-time US-guided CNB. At this time, there are no data on the safety of the US gel if it is introduced into the meninges or the nervous tissues during US-guided regional anesthesia procedures. Therefore, it is difficult to make recommendations; although some clinicians have resorted to using a sterile normal saline solution applied using sterile swabs as an alternative coupling agent to keep the skin moist under the footprint of the transducer. (17) 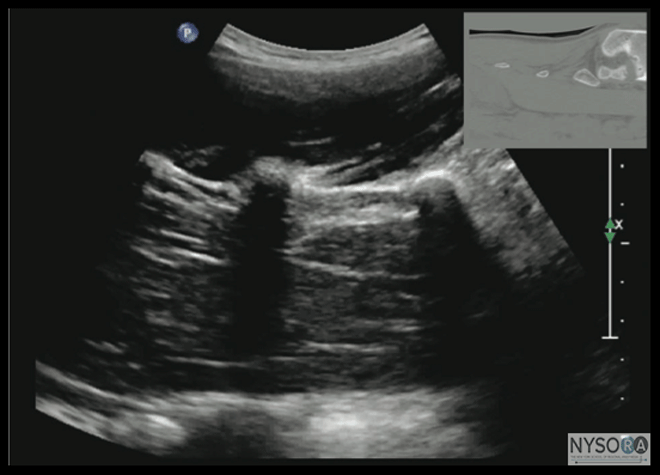
Figure 11: Paramedian sagittal sonogram of the lumbar spine at the level of the transverse processes (TPs). Note the hyperechoic reflections of the TPs with their acoustic shadow that produces the "trident sign." The psoas muscle is seen in the acoustic window between the transverse processes and is recognized by its typical hypoechoic and striated appearance. Part of the lumbar plexus is also seen as a hyperechoic shadow in the posterior part of the psoas muscle between the transverse processes of L4 and L5 vertebra. Picture in the inset shows a corresponding computed tomography (CT) scan of the lumbosacral spine at the level of the transverse processes. The CT slice was reconstructed from a three-dimensional CT data set from the author's archive.
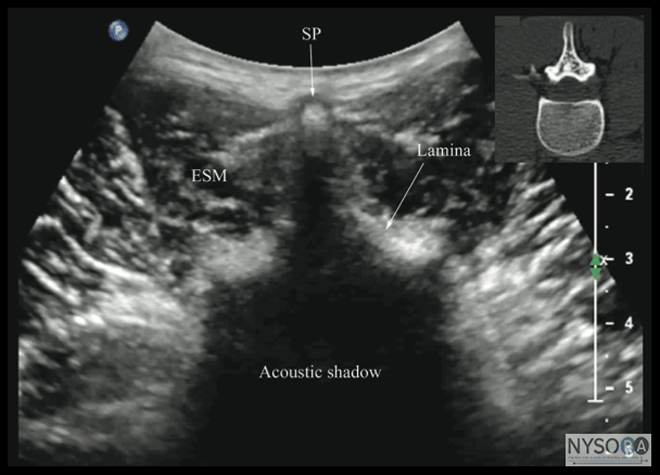
Figure 12: Transverse sonogram of the lumbar spine with the transducer positioned directly over the spinous process (i.e., transverse spinous process view). Note the acoustic shadow of the spinous process and lamina that completely obscures the spinal canal and the neuraxial structures. Picture in the inset shows a corresponding computed tomography (CT) scan of the lumbar vertebra. The CT slice was reconstructed from a three-dimensional CT data set from the author's archive. SP, spinous process; ESM, erector spinae muscle. Spinal Injection There are limited data in the published medical literature on the use of US for spinal (intrathecal) injections, (28,29) although US has been reported to guide lumbar punctures by radiologists (30) and emergency physicians. (31) Most available data are anecdotal case reports. (28,29,32-34) Yeo and French, in 1999, were the first to describe the successful use of US to assist spinal injection in a patient with abnormal spinal anatomy. (34) They used US to locate the vertebral midline in a parturient with severe scoliosis with Harrington rods in situ. (34) Yamauchi and colleagues describe using US to preview the neuraxial anatomy and measure the distance from the skin to the dura in a postlaminectomy patient before the intrathecal injection was performed under X-ray guidance. (33) Costello and Balki described using US to facilitate spinal injection by locating the L5-S1 gap in a parturient with poliomyelitis and previous Harrington rod instrumentation of the spine. (28) Prasad and colleagues report using US to assist spinal injection in a patient with obesity, scoliosis, and multiple previous back surgeries with instrumentation. (29) More recently, Chin and colleagues (32) described real-time US-guided spinal anesthesia in two patients with abnormal spinal anatomy (one had lumbar scoliosis and the other had undergone spinal fusion surgery at the L2-3 level). Lumbar Epidural Injection US imaging can be used to preview the underlying spinal anatomy (2-4) or to guide the Tuohy needle in real time (17) during a lumbar epidural access. Moreover, real-time US guidance for epidural access can be performed by a single (17) or two (4) operators. In the latter technique, described by Grau and colleagues (4) for combined spinal epidural anesthesia, one operator performs the US scan via the paramedian axis while the other carries the needle insertion through the midline approach using a "loss-of-resistance" technique. (4) Using this approach, Grau and colleagues reported the ability to visualize the advancing epidural needle despite different axes of the US scan and needle insertion. (4) They were able to visualize the dural puncture in all patients, as well as dural tenting in a few cases, during the needle-through-needle spinal puncture. (4) 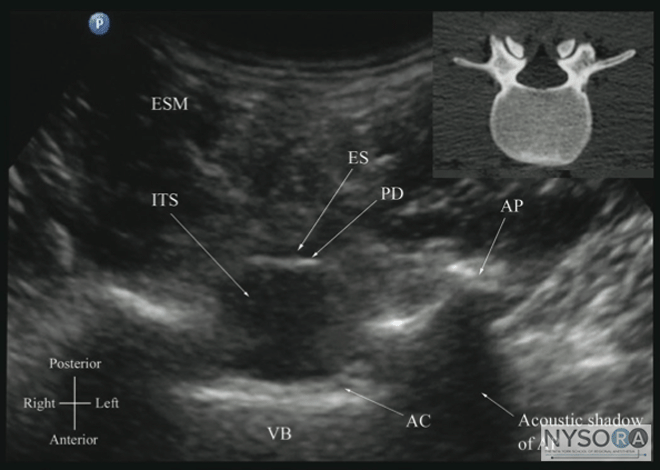
Figure 13: Transverse sonogram of the lumbar spine with the transducer positioned such that the ultrasound beam is insonated through the interspinous space (i.e., transverse interspinous view). The epidural space, posterior dura, intrathecal space, and the anterior complex are visible in the midline, and the articular process (AP) is visible laterally on either side of the midline. Note how the articular processes on either side are symmetrically located. Picture in the inset shows a corresponding computed tomography (CT) scan of the lumbar vertebra. The CT slice was reconstructed from a three-dimensional CT data set from the author's archive. ESM, erector spinae muscle; ES, epidural space; PD, posterior dura; ITS, intrathecal space; AC, anterior complex; VB, vertebral body. Karmakar and colleagues recently described a technique of real-time, US guided epidural injection in conjunction with loss of resistance (LOR) to saline. (17) The epidural access was performed by a single operator, and the epidural needle was inserted in the plane of the US beam via the paramedian axis. (17) Generally, it is possible to visualize the advancing epidural needle in real time until it engages in the ligamentum flavum. (17) The need for a second operator to perform the LOR can be circumvented by using a spring-loaded syringe (e.g., Episure AutoDetect syringe, Indigo Orb, Inc., Irvine, CA, USA), with an internal compression spring that applies constant pressure on the plunger (Figure 18). (17) Anterior displacement of the posterior dura and widen- ing of the posterior epidural space are the most frequently visualized changes within the spinal canal. Compression of the thecal sac can be seen occasionally. (17) These ultrasonographic signs of a correct epidural injection were previously described in children. (35) The neuraxial changes that occur within the spinal canal following the "loss of resistance" to saline may have clinical significance. (17) Despite the ability to use real-time US for establishing epidural access, visualization of an indwelling epidural catheter in adults proved to be more challenging. Occasionally, anterior displacement of the posterior dura and widening of the posterior epidural space after an epidural bolus injection via the catheter can be observed and can be used as a surrogate marker of the location of the catheter tip. Grau and colleagues postulated that this may be related to the small diameter and poor echogenicity of conventional epidural catheters. (15) It remains to be seen whether or not the imminent development of echogenic needles and catheters will have an impact on the ability to visualize epidurally placed catheters. 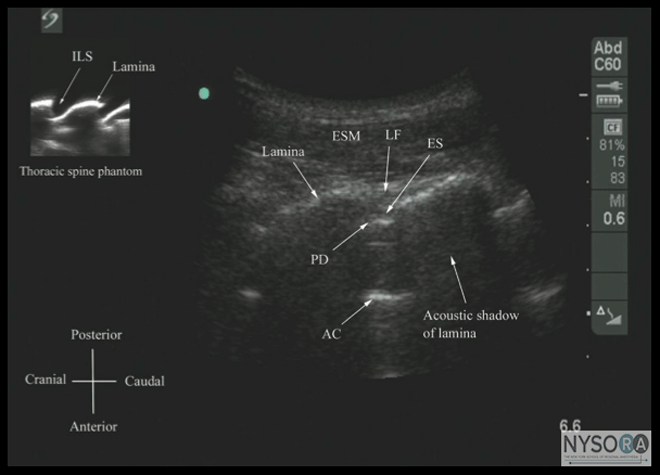
Figure 14: Paramedian oblique sagittal sonogram of the lower-thoracic spine. Note the narrow acoustic window through which the ligamentum flavum (LF), posterior dura (PD), epidural space (ES), and anterior complex (AC) are visible. Picture in the inset shows a sagittal sonogram of the thoracic spine from the water-based spine phantom. ILS, interlaminar space. Thoracic Epidural Injection There are no published data on USG thoracic epidural blocks. This lack may be due to the poor US visibility of the neuraxial structures in the thoracic region compared with the lumbar region (see previous section) and the associated technical difficulties. However, despite the narrow acoustic window, the lamina, the interlaminar space, and the posterior dura are visualized consistently when using the paramedian axis (Figures 14 and 15). The epidural space is more difficult to delineate, but it also is best visualized in a paramedian scan. (13) As a result, a US-assisted technique can be used to perform thoracic epidural catheterization via the paramedian window. In this approach, the patient is positioned in the sitting position and a PMOSS is performed at the desired thoracic level with the orientation marker of the transducer directed cranially. Under strict aseptic precautions (described previously) the Tuohy needle is inserted via the paramedian axis in real time and in the plane of the US beam. The needle is advanced steadily until it contacts the lamina or enters the interlaminar space. At this point, the US transducer is removed and a traditional loss of resistance to saline technique is used to access the epidural space. Because the lamina is relatively superficial in the thoracic region, it is possible to visualize the advancing Tuohy needle in real time. Preliminary experience with this approach indicates that US may improve the likelihood of thoracic epidural access on the first attempt. However, more research to compare the US-assisted technique as described with the traditional approach is necessary before more definitive recommendations on the utility and safety of US for this indication can be made. 
Figure 15: Paramedian oblique sagittal sonogram of the midthoracic spine. The posterior dura (PD) and the anterior complex (AC) are visible through the narrow acoustic window. Picture in the inset shows a corresponding computed tomog- raphy (CT) scan of the midthoracic spine. The CT slice was reconstructed from a three-dimensional CT data set from the author's archive. ILS, interlaminar space; LF, ligamentum flavum. Caudal Epidural Injection For an USG caudal epidural injection, a transverse (Figure 16) or sagittal (Figure 17) scan is performed at the level of the sacral hiatus. Because the sacral hiatus is a superficial structure, a high-frequency (13-6 MHz) linear array transducer is used for the scan as described previously. The needle can be inserted in the short (out-of-plane) or long axis (in-plane). For a long-axis needle insertion, a sagittal scan is performed and the passage of the block needle through the sacrococcygeal ligament into the sacral canal is visualized in real time (Figure 19). However, because the sacrum impedes the travel of the US, there is a large acoustic shadow anteriorly, which makes it impossible to visualize the tip of the needle or the spread of the injectate within the sacral canal. An inadvertent intravascular injection, which reportedly occurs in 5 to 9% of procedures, cannot be detected using US. As a result, the clinician still should factor in traditional clinical signs such as the "pop" or "give" as the needle traverses the sacrococcygeal ligament, ease of injection, absence of subcutaneous swelling, "whoosh test," nerve stimulation, or assessing the clinical effects of the injected drug to confirm the correct needle placement. Chen and colleagues reported a 100% success rate in placing a caudal needle under US guidance as confirmed by contrast fluoroscopy. (25) This report is encouraging, considering that even in experienced hands, failure to place a needle in the caudal epidural space successfully is as high as 25%. (25,36) More recently, Chen and colleagues (37) described using US imaging as a screening tool during caudal epidural injections. (37) In their cohort of patients, the mean diameter of the sacral canal at the sacral hiatus was 5.3 + 2 mm and the distance between the sacral cornua (bilateral) was 9.7 + 1.9 mm. (37) These researchers also identified that the presence of sonographic features such as a closed sacral hiatus and a sacral canal diameter of around 1.5 mm are associated with a greater probability for failure. (37) Based on the published data, it can be concluded that US guidance, despite its limitation, can be useful as an adjunct tool for caudal epidural needle placement and has the potential to improve technical outcomes and minimize failure rates and exposure to radiation in the chronic pain setting, and therefore it deserves further investigation 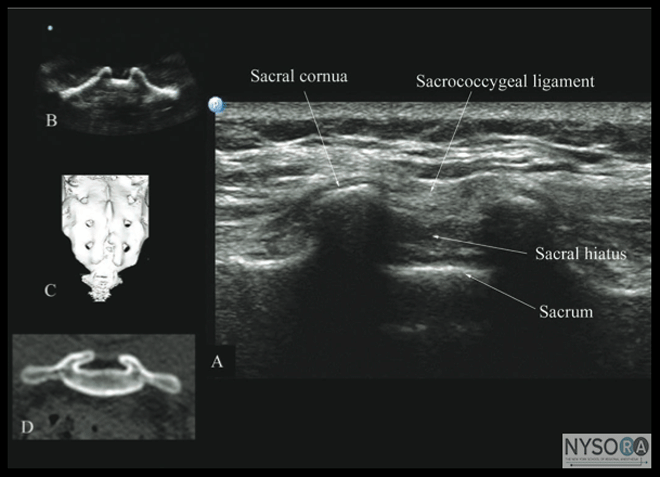
Figure 16: Transverse sonogram of the sacrum at the level of the sacral hiatus. Note the two sacral cornua and the hyperechoic sacrococcygeal ligament that extends between the two sacral cornua. The hypoechoic space between the sacrococcygeal ligament and the posterior surface of the sacrum is the sacral hiatus. Figures in the inset (B) shows the sacral cornua from the water-based spine phantom, (C) shows a three-dimensional (3D) reconstructed image of the sacrum at the level of the sacral hiatus from a 3D CT data set from the author's archive, and (D) shows a transverse CT slice of the sacrum at the level of the sacral cornua. Clinical Utility of Ultrasound for Central Neuraxial Blocks Outcome data on the use of US for CNB are limited and have primarily focused on the lumbar region. Most studies to date evaluated the utility of an out-of-plane prepuncture US scan or scout scan. A scout scan allows the operator to identify the midline (23) and accurately determine the interspace for needle insertion, (16,17,210 which are useful in patients in whom anatomic landmarks are difficult to palpate, such as in those with obesity, (1,38) edema of the back, or abnormal anatomy (scoliosis, (1,39) postlaminectomy surgery, (33) or spinal instrumentation). (28,29,34) It also allows the operator to preview the neuraxial anatomy, (2-4,17,40) identify asymptomatic spinal abnormalities such as in spina bifida, (41) predict the depth to the epidural space, (2,3,9,10) particularly in obese patients, (26) identify ligamentum flavum defects, (42) and determine the optimal site and trajectory for needle insertion. (3,15) Cumulative evidence suggests that a US examination performed before the epidural puncture improves the success rate of epidural access on the first attempt, (2) reduces the number of puncture attempts (1-4) or the need to puncture multiple levels, (2-4) and also improves patient comfort during the procedure. (3) A scan can be useful in patients with presumed difficult epidural access, such as in those with a history of difficult epidural access, obesity, and kyphosis or scoliosis of the lumbar spine. (1) When used for obstetric epidural anesthesia, US guidance was reported to improve the quality of analgesia, reduce side effects, and improve patient satisfaction. (1,4) A scout scan may also improve the learning curve of students for epidural blocks in parturients. (14) Currently, there are limited data on the utility of real-time US guidance for epidural access, (4,17) although the preliminary reports indicate it may improve technical outcomes. (4) 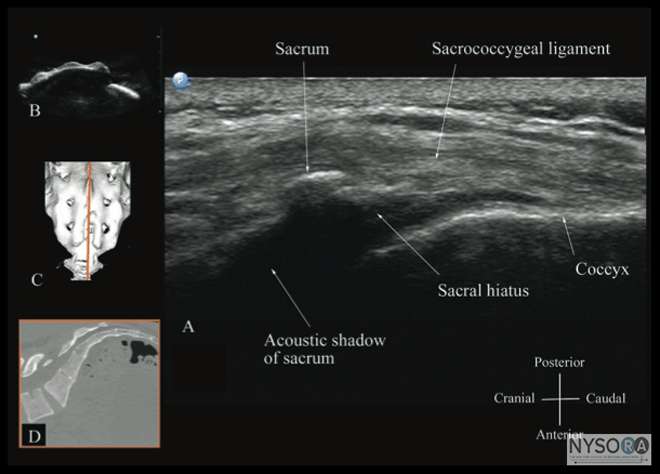
Figure 17: Sagittal sonogram of the sacrum at the level of the sacral hiatus. Note the hyperechoic sacrococcygeal ligament that extends from the sacrum to the coccyx and the acoustic shadow of the sacrum that completely obscures the sacral canal. Figures in the inset: (B) shows the sacral hiatus from the water-based spine phantom, (C) shows a three-dimensional (3D) reconstructed image of the sacrum at the level of the sacral hiatus from a 3D CT data set from the author's archive, and (D) shows a sagittal CT slice of the sacrum at the level of the sacral cornua. Education and Training Learning US-guided CNB techniques takes time and patience. Regardless of the technique used, US-guided CNB and, in particular, real-time US-guided CNB, are advanced techniques and are by far the most difficult USG interventions. Likewise, peri-centroneuraxial blocks, such as lumbar plexus and paravertebral blocks, also demand a high degree of manual dexterity, hand–eye coordination, and an ability to conceptualize two-dimensional information into a 3D image. Therefore, before attempting to perform a US-guided CNB or peri-centroneuraxial blocks, the operator should have knowledge of the basics of US, be familiar with the sonoanatomy of the spine and lumbar plexus, and have the necessary interventional skills. It is advisable to start by attending a course or workshop tailored for this purpose where the operator can learn the basic scanning techniques, spinal sonoanatomy, and the interventional skills. More experience in spinal sonography can also be acquired by scanning human volunteers. (43) 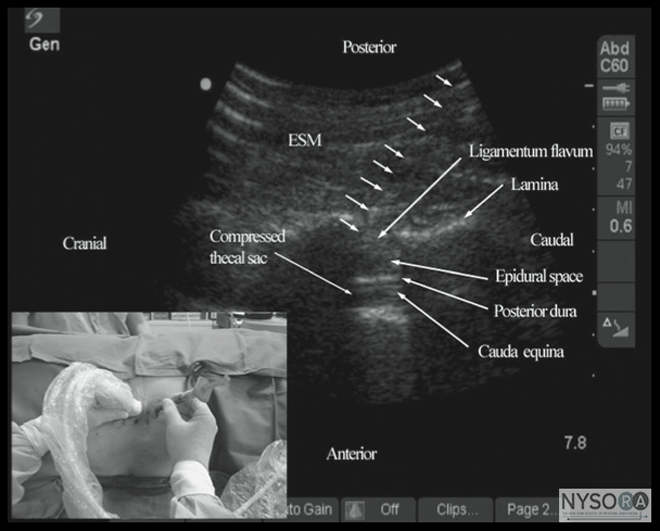
Figure 18: Paramedian oblique sagittal sonogram of the lumbar spine showing the sonographic changes within the spinal canal after the "loss of resistance" to saline. Note the anterior displacement of the posterior dura, widening of the posterior epidural space, and compression of the thecal sac. The cauda equina nerve roots are also now better visualized within the compressed thecal sac in this patient. Picture in the inset shows how the Episure AutoDetect syringe was used to circumvent the need for a third hand for the 'loss of resistance'. Today, there are several models (phantoms) for practicing US-guided central neuraxial interventions. The "water-based spine phantom" (18) is useful for learning the osseous anatomy of the spine, but it is not a good model for learning US-guided spinal interventions because it lacks tissue mimicking properties. Spinal and paraspinal sonography is often taught at workshops, but they are not suitable for prac- ticing actual techniques. Fresh cadaver courses are available, and they allow participants to study the neuraxial sonoanatomy and practice US-guided CNB with realistic haptic feedback, but they may be limited by the quality of the US images. However, such courses are uncommon and conducted in anatomy departments with the cadavers in a position that rarely mimics what is practiced in the operating room. Anesthetized pigs can also be used, but animal ethics approval is required and, for the organizers, a license from the local health department to conduct such workshops. They entail infectious precautions, and religious beliefs may preclude its use as a model. Moreover, such workshops are conducted in designated animal laboratories that are typically small and not suited to accommodate large groups of participants. To circumvent some of these problems, the group at the Chinese University Hong Kong recently introduced the "pig carcass spine phantom," (Figure 4), (19) an excellent model that can be used in conference venues and provides excellent tactile and visual feedback. (19) The limitation of the "pig carcass spine phantom" is that it is a decapitated model and there is loss of cerebrospinal fluid during the preparation process. This presentation results in air artifacts and loss of contrast within the spinal canal during spinal sonography unless the thecal sac is cannulated at its cranial end and continuously irrigated with fluid (normal saline), a process that requires surgical dissection to isolate the thecal sac. Therefore, an "in vitro" model that can facilitate the learning of the scanning techniques and the hand-eye coordination skills required for real-time US-guided CNB is highly desirable. A low-cost gelatin-based US phantom of the lumbosacral spine recently was proposed. (44) However, the gelatin phantom is soft in consistency, lacks tissue-mimicking echogenic properties, does not provide a haptic feedback, is easily contaminated with mold and bacteria, and needle track marks limit its usefulness, (44) all of which preclude its extended use. Karmakar and colleagues recently developed a "gelatin-agar spine phantom" that overcomes some of the drawbacks of the gelatin-based spine phantom. It is mechanically stable, has a tissue-like texture and echogenicity, needle track marks are less of a problem, and it can be used over extended periods of time to study the osseous anatomy of the lumbosacral spine and to practice the hand-eye coordination skills required to perform US-guided CNB. (45) 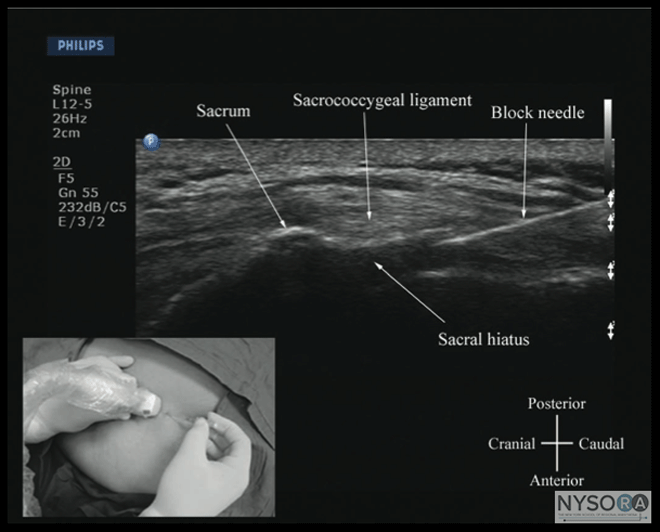
Figure 19: Sagittal sonogram of the sacrum at the level of the sacral hiatus during a real-time ultrasound-guided caudal epidural injection. Note the hyperechoic sacrococcygeal ligament and the block needle that has been inserted in the plane (in-plane) of the ultrasound beam. Picture in the inset shows the position and orientation of the transducer and the direction in which the block needle was inserted. Once the basic skills are attained, it is best to start by performing US-guided spinal injections, under supervision, before progressing to performing epidural blocks. Real-time US-guided epidurals can be technically challenging, even for an experienced operator. If there is no experience in US-guided CNB locally, it is advisable to visit a center where such interventions are practiced. At this time, there is no knowledge of the length of the learning curve for US-guided CNB or how many such interventions are needed to become proficient in performing real-time US-guided CNB. Further research in this area is warranted. Conclusion US-guided CNB is a promising alternative to traditional landmark-based techniques. It is noninvasive, safe, simple to use, can be quickly performed, does not involve exposure to radiation, provides real-time images, and is free from adverse effects. Experienced sonographers are able to visualize neuraxial structures with satisfactory clarity using US, and the understanding of spinal sonoanatomy continues to be clarified. A scout scan allows the operator to preview the spinal anatomy, identify the midline, locate a given intervertebral level, accurately predict the depth to the epidural space, and determine the optimal site and trajectory for needle insertion. Use of US also improves the success rate of epidural access on the first attempt, reduces the number of puncture attempts or the need to puncture multiple levels, and improves patient comfort during the procedure. It is an excellent teaching tool for demonstrating the anatomy of the spine and improves the learning curve of epidural blocks in parturients. US guidance also may allow the use of CNB in patients who in the past may have been considered unsuitable for such procedures due to abnormal spinal anatomy. However, US guidance for CNB is still in its early stages of development, and evidence to support its use is sparse. The initial experience suggests that US-guided CNB is technically demanding, and, therefore, unlikely to replace traditional methods of performing CNB in the near future because traditional methods are well established as simple, safe, and effective in most patients. We envision that as US technology continues to improve and as more anesthesiologists embrace it and acquire the skills necessary to perform US-guided interventions, US-guided CNB may become the standard of care in the future.    |
| 02/20/2016(+ 2016 Dates) | |
| 01/27/2016 | |
| 03/17/2016 | |
| 03/23/2016 (+ 2016 Dates) | |
| 04/20/2016 | |
| 09/23/2016 | |
| 10/01/2024 |
![[advertisement] gehealthcare](../../../files/banners/banner1_250x600/GEtouch(250X600).gif)


























Post your comment