Spinal Anesthesia - continued
|
Technique of Lumbar Puncture When performing a spinal anesthetic, appropriate monitors should be placed, and airway and resuscitation equipment should be readily available. All equipment for the spinal blockade should be ready for use, and all necessary medications should be drawn up prior to positioning the patient for spinal anesthesia. Adequate preparation for the spinal reduces the amount of time needed to perform the block and assists with making the patient comfortable. Proper positioning is the key to making the spinal anesthetic quick and successful. Once the patient is correctly positioned, the midline should be palpated. The iliac crests are palpated, and a line is drawn between them in order to find the body of L4 or the L4-5 interspace. Other interspaces can be identified, depending on where the needle is to be inserted.
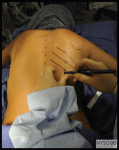
The skin should be cleaned with sterile cleaning solution, and the area should be draped in a sterile fashion. A small wheal of local anesthetic is injected into the skin at the site of insertion. More local anesthetic is then administered along the intended path of the spinal needle insertion to a depth of 1 to 2 in. This serves a dual purpose: additional anesthesia for the spinal needle insertion and identification of the correct path for spinal needle placement. Midline Approach If the midline approach is used, palpate the desired interspace and inject local anesthetic into the skin and subcutaneous tissue. The introducer needle is placed with a slight cephalad angle of 10 to 15 degrees. Next the spinal needle is passed through the introducer. The needle goes through the subcutaneous tissue, supraspinous ligament, interspinous ligament, ligamentum flavum, epidural space, dura mater, and subarachnoid mater in order to reach the subarachnoid space. Resistance changes as the spinal needle passes through each level on the way to the subarachnoid space. Muscle has less resistance to the spinal needle than ligaments. When the spinal needle goes though the dura mater, a “pop” is often appreciated. Once this pop is felt, the stylet should be removed from the introducer to check for flow of CSF. For spinal needles of small gauge (26-29 gauge), this usually takes 5-10 sec, but in some patients, it can take a minute or longer. If there is no flow, the needle might be obstructed by and rotating it 90 degrees may be helpful. Debris can obstruct the orifice of the spinal needle and, if necessary, withdraw the needle and clear the orifice before attempting the spinal anesthetic again. Finally, the spinal needle may not be in the correct position if CSF does not flow freely and the needle should be repositioned. Sometimes the needle hits bone when attempting a spinal anesthetic. When this occurs, note the depth of the needle at bone contact, and place the needle more cephalad. If bone is contacted on reinsertion of the needle, the depth should be compared. If the needle contacts bone deeper on reinsertion, then likely the inferior spinous process is being contacted and the needle should be inserted even more cephalad. If the bone contact is shallower, then likely the superior spinous process is being felt and the needle needs to be redirected in a caudal fashion. If the depth of bone contact is the same, then likely the vertebral lamina is being contacted and the needle is off the midline. Palpation and identification of the midline before reinsertion would be advisable.
When the spinal needle needs to be reinserted, it is important to withdraw the needle back to the skin without removing it before redirection. Only make small changes in the angle of direction when reinserting the spinal needle as even small changes at the surface can lead to large changes in direction when the needle reaches the meninges. Bowing and curving of the spinal needle when inserting through the skin can steer the needle off course when attempting to contact the subarachnoid space. Paresthesias may be elicited when passing a spinal needle. If the needle tip encounters a nerve root, the patient may feel a paresthesia. The stylet should be removed from the spinal needle, and if CSF is seen and the paresthesia no longer present, it is safe to inject the local anesthetic. Most likely a cauda equina nerve root was encountered. If there is no CSF flow, most likely the spinal needle has contacted a spinal nerve root traversing the epidural space. The needle should be removed and redirected toward the side opposite the paresthesia. Sometimes, needle contact with bone can be interpreted as a paresthesia and in this case, the spinal needle should be redirected. After free flow of CSF is established, inject the local anesthetic smoothly and slowly at a speed of less than 0.5 mL/sec. Additional aspiration of CSF at the midpoint and end of injection should be attempted to confirm continued subarachnoid administration but may not always be possible when small needles are used. Once local anesthetic injection is complete, the introducer and spinal needle are removed as one unit from the back of the patient. The patient should then be positioned according to the surgical procedure and baricity of local anesthetic given. The table can be tilted either in the Trendelenburg or reverse Trendelenburg position as needed to adjust the height of the block after testing the sensory level. The anesthesiologist should carefully monitor and support vital signs. Paramedian (Lateral) Approach If the patient has a heavily calcified interspinous ligament or difficulty in flexing the spine, a paramedian approach to achieve spinal anesthesia can be utilized. The patient can be in any position for this approach: sitting, lateral, or even prone jackknife. After identifying the correct level for spinal anesthesia placement, the spinous process is palpated. The needle should be inserted 1 cm lateral to this point and directed toward the middle of the interspace. The ligamentum flavum is usually the first resistance identified, but sometimes the lamina is contacted. If this is the case, redirection of the needle should be performed.
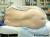
Another method is to insert the needle 1 cm lateral and 1 cm inferior to the interspace and contact the lamina. After the bone is contacted, the needle should be walked off the lamina and into the subarachnoid space. Figure 13 shows the landmarks used for a paramedian approach to spinal anesthesia. Figure 14 depicts successful performance of a paramedian spinal anesthetic. 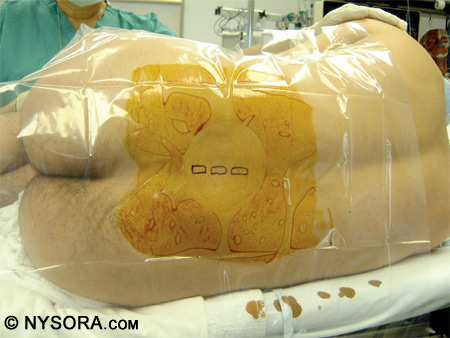
Figure 13: The landmarks used for a paramedian approach to spinal anesthesia. The patient is in the right lateral decubitus position and the spinous processes are marked. 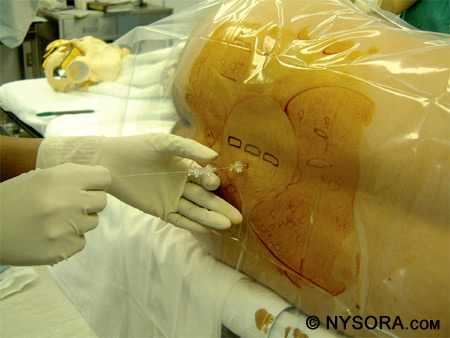
Figure 14: Successful performance of a paramedian spinal anesthetic. The needle is inserted 1 cm lateral to the spinous process and directed toward the middle of the interspace. Taylor Approach The Taylor, or lumbosacral, approach to spinal anesthesia is a paramedian approach directed toward the L5-S1 interspace. Due to the fact that this is the largest interspace, the Taylor approach can be used when other approaches are not successful or cannot be performed. As with the paramedian approach, the patient can be in any position for this approach: sitting, lateral, or prone.
The needle should be inserted at a point 1 cm medial and inferior to the posterior superior iliac spine, then angled cephalad 45-55 degrees. This should be medial enough to reach the midline at the L5 spinous process. After needle insertion, the first significant resistance felt is the ligamentum flavum, and then the dura mater is punctured to allow free flow of CSF as the subarachnoid space is entered. Figure 15 shows the Taylor approach to spinal anesthesia. 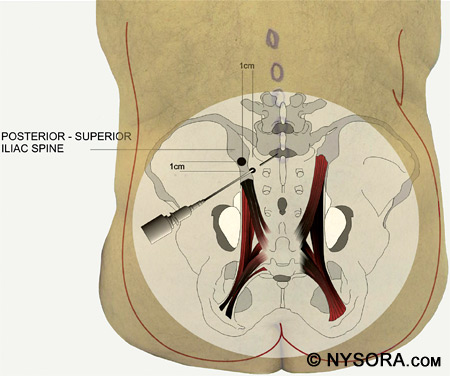
Figure 15: The Taylor approach to spinal anesthesia. The needle is inserted 1 cm medial and inferior to the posterior superior iliac spine, then angled cephalad 45–55 degrees. Continuous Catheter Techniques Sometimes a catheter is placed for continuous spinal anesthesia. Local anesthetics can be dosed repeatedly through the catheter and the level and duration of anesthesia adjusted as necessary for the surgical procedure. Placement of a continuous spinal catheter occurs in a similar fashion as a regular spinal anesthetic except that a larger gauge needle, such as a Tuohy, is used to enable the passage of the catheter. After insertion of the Tuohy needle, the subarachnoid space is found and the spinal catheter is passed 2-3 cm into the subarachnoid space. If there is difficulty in passing the catheter, attempt to rotate the Tuohy needle 180 degrees. Never withdraw the catheter back into the needle shaft because there is a risk of shearing the catheter and leaving a piece of it in the subarachnoid space. If the catheter needs to be withdrawn, withdraw the catheter and needle together, and attempt the continuous spinal at another interspace.
Since the needle used to pass the spinal catheter is a large-bore needle, there is a much higher risk of PDPH, especially in young female patients. Cauda equina syndrome can occur with small spinal catheters, so the FDA has advised against using catheters smaller than 24 gauge for continuous spinal anesthetics.[196-199] Intraoperative & Postoperative Management Depending on the baricity of the local anesthetic solution injected, the level of the spinal anesthetic may be adjusted in the first few minutes, i.e., if a hypobaric or hyperbaric solution is used. After injection of the spinal anesthetic, assess the cardiovascular status of the patient, as changes can occur up to 20 min after spinal anesthesia injection. Continued monitoring of the heart rate and frequent readings of the blood pressure are recommended to detect hypotension so that corrections can be made early and quickly. Intraoperative management of spinal anesthesia is much like intraoperative management with other forms of regional anesthesia. The patient may not feel operative pain but may still be uncomfortable. Supplemental benzodiazepines or opioids can be given intravenously. Comforting words from a caring, empathetic anesthesiologist also go a long way toward putting the patient at ease. Many factors come into play about whether to administer additional intravenous medications.
Elderly patients can become confused with benzodiazepines. Propofol can be given as an infusion to assist with making the patient more comfortable, and opioids can assist with pain from an unanesthetized portion of the body. Oxygen should always be administered via facemask or nasal cannula. Postoperatively the anesthesiologist must continue to monitor the patient until the spinal anesthetic recedes. In most circumstances the patient should void prior to being discharged from the postanesthesia care unit. Since the voiding mechanism is innervated by sacral autonomic fibers, which are the last to return to preoperative function, voiding may be delayed. The risk of discharging a patient from the recovery room prior to voiding includes complications involving a distended or ruptured bladder. Hypotension from continued blood volume redistribution as well as surgical bleeding should be monitored postoperatively. Complications Complications of spinal blockade include local anesthetic neurotoxicity and neurologic injury, PDPH, high spinal blockade, and cardiovascular collapse. Neurotoxicity studies performed in animal models producing neurologic deficits and changes in spinal cord histology are not seen in clinically useful concentrations of tetracaine, lidocaine, bupivacaine, or chloroprocaine in humans. High concentration tetracaine and lidocaine causes histopathologic changes and neurologic deficits in animal models.[200-202] Spinal cord blood flow is increased, and vasodilation occurs with intrathecal bupivacaine, lidocaine, mepivacaine, and tetracaine, but ropivacaine causes vasoconstriction and decreased spinal blood flow in a dose-dependent fashion. TNS may occur with spinal anesthesia, usually with lidocaine. Permanent Neurologic Injury Permanent neurologic injury is a devastating complication after spinal anesthesia. In a large, prospective survey in France, Auroy and colleagues reported 12 neurologic complications when a series of 35,439 spinal anesthetics were reviewed.[203] Nine peripheral neuropathies and three cases of cauda equina were seen, which correlates to a 0.03% neurologic complication rate. Moen and coworkers reported similar complication rates after spinal anesthesia.[204] Neurologic injury can occur after needle introduction into the spinal cord or nerves, spinal cord ischemia, bacterial contamination of the subarachnoid space, or hematoma formation. Although the elicitation of paresthesias during spinal anesthesia has been implicated as a risk factor for persistent neurologic injury, it is not known whether injection of the local anesthetic solution after a paresthesia is elicited should be stopped.[205] It is also unknown as to whether the actual injection of local anesthesia after paresthesia is elicited causes permanent neurologic damage, similar to what happens in peripheral nerves when an injection is associated with high pressures during injection of local anesthetic solution.[206,207] It is possible that when paresthesia occurs, that a spinal nerve has been penetrated by the spinal needle; consequently- injection of local anesthetic into the spinal nerve causes permanent neurologic damage. Cauda Equina Syndrome Cauda equina syndrome is associated with the use of continuous spinal microcatheters.[196-199] The use of hyperbaric 5% lidocaine for spinal anesthesia is also associated with an increased incidence of cauda equina syndrome,[208-210] although other local anesthetics have been found to cause it.[204,211-213] Other risk factors for cauda equina syndrome include repeated dosing of local anesthetic solution through continuous spinal catheters or multiple single-shot spinal anesthetics. Current suggestions for prevention of cauda equina syndrome from spinal anesthesia include aspiration of CSF before and after local anesthetic injection, and if CSF cannot be aspirated after injection, do not inject a full dose of local anesthetic. Evaluation of sacral blockade after spinal injection is important so that distribution of local anesthetic can be documented. Limiting the amount of local anesthetic given in the subarachnoid space, and if a spinal anesthetic has to be repeated, use of a different local anesthetic also may help preventing cauda equina syndrome.
Arachnoiditis Arachnoiditis can occur after spinal injection of local anesthetic solution, but is also known to occur after intrathecal steroid injection.[214-217] Causes of arachnoiditis include infection, myelograms from oil-based dyes, blood in the intrathecal space, neuroirritant, neurotoxic or neurolytic substances, surgical interventions in the spine, intrathecal corticosteroids, and trauma. In regard to spinal anesthesia, arachnoiditis has resulted from traumatic dural puncture, local anesthetics, detergents, antiseptics or other substances unintentionally injected into the spinal canal.[218] Spinal Hematoma Formation Spinal hematoma formation is rare complication after spinal anesthesia and infrequently occurs in the absence of trauma or anticoagulant therapy. Major spontaneous hemorrhagic complications have been reported after antithrombotic and thrombolytic therapy.[219] Risk factors for spinal hematoma formation include the intensity of the anticoagulant effect, increased age, female gender, history of gastrointestinal bleeding, concomitant aspirin use, and length of therapy.[220] Although most spinal hematomas occur in the epidural space due to the prominent epidural venous plexus, very few reports have mentioned subarachnoid bleeding as causing neurologic deficits because of the lack of major blood vessels in this area. The source of the bleeding can be from either an injured artery or vein. If new or progressive neurologic symptoms develop, an immediate neurosurgery consult should be obtained and a magnetic resonance image (MRI) of the spine should be performed as soon as possible. A recent study from Sweden showed that over a 10-year period from 1990 to 1999, out of 1,260,000 spinal anesthetics, eight spinal hematomas occurred, for an incidence of 0.00063%. Seven of the hematomas formed after single-shot spinal anesthesia and one formed after a continuous spinal blockade. A total of 33 spinal hematomas were noted in the study after both epidural and spinal blockade, and of these, 11 patients had evidence of coagulopathy or thromboprophylaxis administered soon before or after the central neuraxial blockade. In 10 patients who had spinal hematoma formation, difficulty in placing the epidural or spinal was noted, and in 5 patients symptoms of spinal hematoma appeared in the immediate postoperative period or shortly after removal of epidural catheter. Fourteen patients had symptoms of spinal hematoma appear from 6 h to 3 days after central neuraxial block was performed, and in one patient, pain and paraparesis appeared 2 weeks after difficult spinal anesthetic placement. Of the 33 patients, 6 had full recovery, but 27 suffered permanent neurologic damage. Five of the six patients that had full recovery were treated conservatively, but one underwent laminectomy. Eleven of the patients who did not recover underwent laminectomy, and in a further six cases, laminectomy was considered, but, due to delay in diagnosis, was not performed. Thirteen patients suffered paraparesis, three had cauda equina syndrome, three were left with sensory deficit, three died, and five were reported only to have lack of recovery without more information.[204] Meningitis Meningitis, either bacterial or aseptic, can occur after spinal anesthesia is performed.[221] Sources of infection include contaminated spinal trays and medication, patient infection, and oral flora from anyone behind the patient without a facemask on. Povidone-iodine solution is most commonly chosen for skin antisepsis before initiation of epidural and spinal anesthesia, and single-use containers are suggested. Most cases of meningitis after spinal anesthesia in the first half of the twentieth century were aseptic and could be traced to chemical contamination and detergents.[222,223] Marinac showed that causes of drug- and chemical-induced meningitis include the nonsteroidal antiinflammatory drugs, certain antibiotics, radiographic agents, and muromonab-CD3. There also appears to be an association between the occurrence of the hypersensitivity-type reactions and underlying collagen vascular or rheumatologic disease.[224] Carp and Bailey performed lumbar puncture (LP) in bacteremic rats, and only those with a circulating Escherichia coli count of greater than 50 CFU/mL at the time of LP developed meningitis.[225] Although meningitis after LP also been described in bacteremic children,[226] the incidence of meningitis after diagnostic LP is not significantly different in bacteremic patients compared with spontaneous incidence of meningitis.[227] Oral flora can contaminate the CSF when a spinal anesthetic is being performed. Streptococcus salivarius, S. viridans, Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter, and Mycobacterium tuberculosis have all been isolated in cases of bacterial meningitis after spinal anesthesia or LP.[228-231] It is of utmost importance to use a strict aseptic technique when performing spinal anesthesia. Anyone behind the patient during spinal anesthetic administration must wear a cap and facemask to prevent seeding of the patient's CSF with oropharyngeal flora. In general, to reduce neurologic complications after spinal anesthesia, some safety rules should be followed. Maintain strict sterility throughout the spinal block procedure. Anyone behind the patient during spinal anesthetic placement must wear a cap and facemask. Check laboratory values of the patient and make sure coagulation parameters are within normal limits. Follow the second ASRA consensus conference guidelines on neuraxial anesthesia and anticoagulation.[232] When giving a spinal anesthetic, use the lowest efficient dose of local anesthetic solution. Incomplete neural blockade should not necessarily lead to another spinal injection of local anesthetic solution, reevaluate and attempt to change the level of the spinal block prior to repeat injection. Avoid large volumes and repeated injections of hyperbaric lidocaine. Never inject preservative-containing solutions into the subarachnoid space. The administration of new compounds in the subarachnoid space must be supported by data of spinal neuropharmacology and the lack of neurotoxicity must have been previously checked with animal studies.[233] Postdural Puncture Headache PDPH was first described by Dr. August Bier in 1898, after experimenting on himself. The incidence of PDPH is up to 25% after spinal anesthesia, and the main morbidity after PDPH is restriction of activities of daily living. The headache is characteristically worse when the head is elevated and becomes milder or completely relieved when the patient is supine. PDPH is due to loss of CSF. The subsequent low CSF pressure causes traction on nerve roots and intracranial structures when the patient stands upright. Pain after dural puncture is probably due to increase in cerebral blood flow (CBF). As CSF pressure decreases, CBF increases in order to maintain a constant intracranial volume. Cranial nerve symptoms such as diplopia and tinnitus may occur along with nausea and vomiting. Incidence of PDPH decreases with increasing age and use of small-diameter needles with noncutting, pencil-point needles.[234-236] Although most patients can be treated conservatively with fluids, caffeine, bed rest, analgesics, and sumatriptan, it can take anywhere from 1 to 6 weeks for symptoms to resolve spontaneously. Epidural blood patch remains the mainstay of invasive treatment for PDPH.[237] Effectiveness of blood patching ranges from 64% in obstetrical patients to 95% in the nonpregnant population. The proposed mechanism of action of blood patching is believed to be clot formation over the meningeal hole, preventing further leakage of CSF while the dural puncture heals. Symptoms usually resolve within 1 to 24 h. In patients who do not have symptomatic relief after the first epidural blood patch, a second epidural blood patch is effective 90% of the time. Complications may also arise after epidural blood patch. Reports of back pain, neck pain, lower extremity pain, transient temperature elevation, cranial nerve palsies, nerve root irritation, seizures, acute mental deterioration, subdural hematoma, permanent paresthesias, and cauda equina syndrome have been noted after an epidural blood patch was performed. The most common complication is back pain, which can occur in up to 35% of patients. However, an epidural blood patch is well tolerated if performed with attention to asepsis.[238] If any acute mental changes occur or a second blood patch fails to produce relief, a neurology consult should be immediately obtained.
Intravenous injection of 500 mg of caffeine results in better relief of PDPH than placebo.[234,239,240] One to two doses intravenously should be adequate. Three hundred milligrams of oral caffeine has also been shown to be better than placebo in relieving PDPH.[241] A cup of 150 mL of coffee contains 150 mg of caffeine. Because caffeine is a cerebral vasoconstrictor and a CNS stimulant, complications may occur after administration, including seizures and transient atrial fibrillation. Sumatriptan is a serotonin agonist and causes cerebral vasoconstriction. Sumatriptan is used for the treatment of migraines; however, there are conflicting reports on the value of sumatriptan for PDPH.[242-244] Caution should be used for patients with coronary artery disease or Prinzmetal angina as sumatriptan can cause coronary artery vasospasm. Since sumatriptan is injected subcutaneously, pain at the injection site may occur. More studies need to be performed to determine the usefulness of sumatriptan in treating PDPH. High Spinal Anesthesia High spinal anesthesia can result in profound respiratory impairment, most likely due to brainstem ischemia. If the blood pressure and cardiac output become too low due to vasodilation, cerebral blood flow can be impaired. This leads to ischemia of the medullary respiratory center. If blood pressure and cardiac output are restored quickly, spontaneous respiration can return almost immediately. This illustrates the importance of monitoring the vital signs closely and acting quickly to correct them. Cardiovascular Collapse Cardiovascular collapse can occur after spinal anesthesia, although it is a rare event. Auroy and coworkers reported only 9 cardiac arrests in 35,439 spinal anesthetics performed.[203] Bradycardia usually precedes cardiac arrest,[143,153,245-247] and early, aggressive treatment of bradycardia is warranted. Treatment of bradycardia includes intravenous atropine, ephedrine, and epinephrine. In cases of cardiac arrest after spinal anesthesia, epinephrine should be used early, and the Advanced Cardiac Life Support (ACLS) protocol should be initiated.[248] There are a few theories as to why spinal anesthesia results in cardiac arrest. Respiratory depression, excessive sedation, and decrease in preload have all been implicated in the past, but they have all been disputed. Hyperventilation, not hypoventilation occurs with spinal block levels up to T4.[249] Cardiac arrest after spinal anesthesia can occur with an oxygen saturation above 95%, and hypoxemia cannot be blamed as the sole cause of cardiac arrest.[153,250] Excessive sedation has also been proposed as a reason for cardiac arrest, but with the use of pulse oximeters, it is unlikely that either excessive sedation or hypoxemia is a cause.[248] A decrease in preload likely leads to increased parasympathetic response that results in bradycardia.[248,251] Activation of three types of receptors have been proposed to cause bradycardia: the low-pressure baroreceptors in the right atrium, the receptors in the myocardial pacemaker, and the mechanoreceptors in the left atrium.[153] Other factors that can lead to more severe bradycardia after spinal anesthesia include high sympathetic blockade from the spinal anesthetic, hypercarbia, hypoxemia, excess sedation from medications given, and chronic medications that cause suppression of the sympathetic nervous system. Outpatient Spinal Anesthesia Each year the number of surgeries increase and more are performed on an outpatient basis. As anesthesiologists,we are always looking for new ways to provide efficient anesthetic care that is safe, controls pain, allows the patient to be discharged home in a timely fashion as per postanesthesia care unit protocol, and is easily performed and reproducible in multiple patients. Spinal anesthesia fits very well into the outpatient surgery model,[253] and techniques and medications from the past are being used again.
In the past, spinal administration of 2-choloroprocaine was associated with chronic neurologic deficits that were believed to be due to sodium bisulfite, an antioxidant used to prolong the shelf life of chloroprocaine.[189,200,254] Trials of spinal 2-chloroprocaine are being performed with new formulations that do not include sodium bisulfite.[186,188,255-260] Forty milligrams of spinal 2-chloroprocaine produces a peak block height of T7, tourniquet tolerance of 46 min, and discharge within 104 min. Forty milligrams of 2% isobaric lidocaine produces a peak block height of T8, tourniquet tolerance of 38 min, and discharge within 134 min. Slightly hyperbaric bupivacaine (1.00100; 7.5 mg) produces a peak block height of T9, tourniquet tolerance of 46 min, and ambulation within 191 min. Table 5 lists various choices of local anesthetics for spinal anesthesia, peak block height level and the duration of anesthesia. Common Clinical Problems & Dilemmas In Practice Of Spinal Anesthesia As with any other form of anesthesia, spinal anesthesia can present clinical problems. This includes inability to locate the subarachnoid space due to difficulties with patient positioning, a lower level of spinal blockade than required for surgery, and the use of spinal anesthesia for outpatient surgery. Whenever there are problems with placing a spinal anesthetic, the anesthesiologist should check the position of the patient. In order to maximize the chances of success, optimal positioning of the patient should be sought. If the patient is in the sitting position, the shoulders should be placed in a down position, arms should be in front of the patient, neck should be flexed, and the lower back should be pushed out posteriorly so that the interspace can be maximally exposed to the anesthesiologist. A member of the operating room personnel who is trained to assist with patient positioning should be used. If the proposed interspace cannot be found by the midline technique, the paramedian technique can be attempted. The interspace above or below the original site of spinal injection can be attempted with adjustment to the local anesthetic that is injected.
Positioning of the patient can also be enhanced with commercially available positioning devices. These devices can help maintain spinal flexion and create a stable support for the personnel are available to assist with positioning. When the sitting position cannot be used or is unsuccessful, the lateral decubitus position can be used. Either the midline or lateral paramedian technique can be attempted. The largest interlaminar space is at L5, and this can be sought via Taylor's approach, which is described previously in this article. Sometimes an insufficient dose of local anesthetic is injected into the subarachnoid space and the level of anesthesia is inadequate for surgery. A repeat injection of local anesthetic could lead to excess spinal blockade and possibly cause high or total spinal anesthesia. The alternatives include general anesthesia, peripheral nerve blockade, or infiltration of local anesthesia at the surgical site by the surgeon along with sedation. Every case can offer different options depending on the health status of the patient, the surgery being performed, use of a tourniquet, and other issues pertaining to the case. It is important to think through what will be best for the patient before choosing another plan of action.
In the past, spinal anesthesia was used sparingly in outpatient surgical procedures because of prolonged recovery room stays after a long-acting local anesthetic was given. Currently, spinal anesthesia is an accepted and sometimes preferred method of providing outpatient anesthesia. Short-acting local anesthetics, such as preservative-free 2-chloroprocaine, have been used with success for surgical procedures lasting an hour or less with no reports of TNS after surgery.[255] Recent Developments In Spinal Anesthesia Use of a unilateral spinal block for elderly patients and outpatient surgery has recently come into vogue. Unilateral spinal anesthesia was described in 1950 by Ruben and Kamsler. They reported 116 patients for surgical reduction of hip fracture performed under unilateral spinal blockade.[261] No deaths were reported and no increase in the hazard of operation was found. Recently, attention has returned to the use of unilateral spinal anesthesia in elderly patients[262] and for outpatient surgery.[263] Elderly patients frequently present with femoral neck fractures and changes in blood pressure can be deleterious. Use of unilateral spinal anesthesia has shown no significant changes in systolic, mean and diastolic pressures, or oxygen saturation in elderly trauma patients. It is recommended that a fascia iliaca block be performed, keeping the operative side up and using a hypobaric spinal solution in low dose for these cases. Outpatient surgery using hyperbaric 0.5% bupivacaine takes about 16 minutes for surgical anesthesia from time of injection for unilateral spinal anesthesia and 13 minutes with traditional bilateral spinal anesthesia. Less hemodynamic changes are found in the unilateral spinal anesthesia group with quicker regression of the block and equal time to discharge home.[264] In performing a unilateral spinal anesthesia, use of a Whitacre 25-gauge or 27-gauge needle with the bevel opening directed at the operative side is suggested. Low-dose bupivacaine should be used, with hyperbaric bupivacaine in outpatient surgery and hypobaric bupivacaine in the elderly trauma patient.[265] A slow injection rate should be used to produce laminar flow that will assist in producing a unilateral blockade. There is little evidence that keeping a patient in the lateral position for more than 15 min is helpful.
As the population of older patients increases, novel methods of preventing deep venous thrombosis (DVT) and keeping these patients anticoagulated have been developed. Reports of spontaneous spinal and epidural hematoma formation were noted in the cardiology and neurosurgery literature in patients who were anticoagulated.[266-270] As spinal anesthesia was found to be useful in controlling pain postoperatively, such as in lower leg amputations, concern arose over complications occurring in such patients who may have been on concomitant anticoagulant therapy.[271-275] The concerns of performing central neuraxial block on anticoagulated patients led to the second ASRA consensus conference on neuraxial anesthesia and anticoagulation.[232] There is a very limited risk of spinal hematoma when performing spinal anesthesia while a patient is on subcutaneous heparin. After LMWH administration, spinal anesthesia should be delayed 10-12 h. If blood is noted during needle placement, LMWH therapy should be delayed 24 h. In cases of continuous spinal anesthesia and accidental LMWH therapy, the catheter should be removed 10-12 h after the last dose of LMWH. Spinal anesthesia should not be performed for 14 days after the last dose of ticlopidine and 7 days after clopidogrel. Patients should not receive glycoprotein IIb/IIIa inhibitors for 4weeks after surgery, and spinal anesthesia should not be attempted until platelet function returns to normal. Many patients ingest herbal medications, and currently there are no specific concerns regarding spinal anesthesia in these patients. |
| 03/23/2016 (+ 2016 Dates) | |
| 04/09/2024(+ 2016 Dates) | |
| 04/20/2016 | |
| 09/23/2016 | |
| 10/01/2024 | |
| 11/03/2024 |
![[advertisement] gehealthcare](files/banners/banner1_250x600/GEtouch(250X600).gif)






















Post your comment