Spinal Anesthesia - continued
|
Pharmacodynamics Of Spinal Anesthesia The pharmacodynamics of spinal injection of local anesthesia are wide-ranging. The next section reviews the cardiovascular, respiratory, and gastrointestinal consequences of spinal anesthesia. This portion of the chapter focuses on the hepatic and renal effects of spinal anesthesia. Hepatic blood flow correlates to arterial blood flow. There is no autoregulation of hepatic blood flow, thus, as arterial blood flow decreases after spinal anesthesia, so does hepatic blood flow.[123] If the anesthesiologist maintains mean arterial pressure (MAP) after placing a spinal anesthetic, hepatic blood flow will be maintained. Patients with hepatic disease must be carefully monitored and their blood pressure must be controlled during anesthesia to maintain hepatic perfusion. No studies have conclusively shown the superiority of regional or general anesthesia in patients with liver disease.[124-128] In patients with liver disease either regional or general anesthesia can be given, as long as the MAP is kept close to baseline.
Renal blood flow is autoregulated. The kidneys remain perfused when the MAP remains above 50mmHg. Transient decreases in renal blood flow may occur when MAP is less than 50 mm Hg, but even after long decreases in MAP, renal function returns to normal when blood pressure returns to normal. Again, attention to blood pressure is important after placing a spinal anesthetic, and the MAP should be as close to baseline as possible. Spinal anesthesia does not affect autoregulation of renal blood flow. It has been shown in sheep that renal perfusion changed very little after spinal anesthesia.[129-132] Cardiovascular Effects of Spinal Anesthesia The sympathectomy produced by spinal anesthesia induces hemodynamic changes. The block height determines the extent of sympathetic blockade, which determines the amount of change in cardiovascular parameters. However, this relationship cannot be predicted. Hypotension and bradycardia are the most common side effects seen with sympathetic denervation.[133] Risk factors associated with hypotension include hypovolemia, preoperative hypertension, high sensory block height, age older than 40 years, obesity, combined general and spinal anesthesia, and addition of phenylephrine to the local anesthetic.[134-136] Chronic alcohol consumption, history of hypertension, elevated BMI, high level of sensory block height, and urgency of surgery all increase the likelihood of hypotension after spinal anesthesia.[137] Hypotension occurs in about33%of the non-obstetric population.[134] Figure 7 depicts changes in blood pressure and heart rate after injection of hyperbaric bupivacaine and tetracaine.[138] 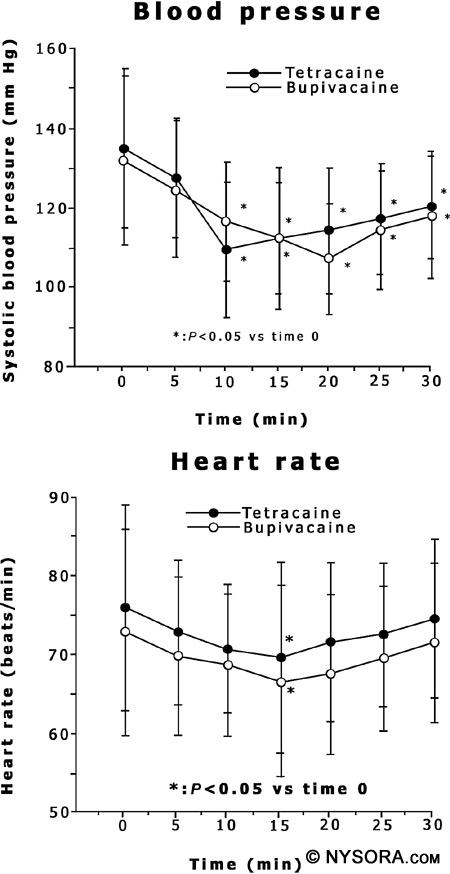
Figure 7: A graph depicting changes in blood pressure and heart rate after injection of hyperbaric bupivacaine and tetracaine. Blood pressure is shown in the upper graph and heart rate is shown in the lower graph with the mean ± SD. Time 0 is the time before spinal anesthetic placement and time 5 is 5 minutes after spinal anesthetic placement. Reproduced with permission from Nishiyama T, Komatsu K, Hanaoka K.: Comparison of hemodynamic and anesthetic effects of hyperbaric bupivacaine and tetracaine in spinal anesthesia. J Anesth., 17:219, 2003. Arterial and venodilation both occur in spinal anesthesia and combine to produce hypotension. Arterial vasodilation is not maximal after spinal blockade, and vascular smooth muscle continues to retain some autonomic tone after sympathetic denervation. Due to retention of autonomic tone, total peripheral vascular resistance (TPVR) decreases only by 15% to 18%, thus MAP decreases by 15% to 18% if cardiac output is not decreased. In patients with coronary artery disease, systemic vascular resistance can be decreased by up to 33% after spinal anesthesia.[139] However, after spinal anesthesia, venodilation will be maximal, depending on the location of the veins. If the veins lie below the right atrium, gravity will cause pooling of the blood peripherally, and if the veins are above, there is back-flow of the blood into the heart. Venous return to the heart, or preload, therefore depends on patient positioning during spinal anesthesia.[140]
Because preload determines cardiac output and patient positioning is a major factor in determining preload, as long as a euvolemic patient is positioned with the legs elevated above the heart, there should be no significant changes in cardiac output after spinal anesthesia. The reverse Trendelenburg position, however, leads to large decreases in preload and thus large decreases in cardiac output.[141,142] Most patients do not experience a significant change in heart rate after spinal anesthesia, but in young (age < 50), healthy (ASA class 1) patients there is a higher risk of bradycardia. Beta-blocker use also increases the risk of bradycardia. The incidence of bradycardia in the nonpregnant population is about 13%.[134] The sympathetic cardiac accelerator fibers emerge from the T1 to T4 spinal segments, and blockade of these fibers is proposed as the cause of bradycardia. Decreased venous return may also cause bradycardia, due to a fall in filling pressures. This triggers the intracardiac stretch receptors to lower the heart rate. Even though both of these mechanisms are proposed to cause bradycardia, other as yet undetermined factors may contribute to the bradycardia seen with spinal anesthesia.[143] Even though bradycardia is usually well tolerated, asystole and second- and third-degree heart block can occur, so it is wise to be vigilant when monitoring a patient after spinal anesthesia and treat promptly and aggressively.[144] Hypotension occurs in about 33% of the nonobstetric population.[134] Treatment of Hypotension After Spinal Anesthesia To effectively treat hypotension, the cause of the hypotension must be corrected. Decreased cardiac output and venous return must be treated, and a bolus of crystalloid is often used to enhance venous volume. The practice of prehydration with 500 to 1500 mL of crystalloid has been shown to decrease hypotension in some studies, but not in others.[145-147] No reliable method to prevent hypotension after spinal blockade exists. Treatment of hypotension, however, remains essential so that the myocardium and brain remain perfused. If a patient is asymptomatic, decreases in blood pressure up to 33% need not be treated. Careful monitoring of blood pressure as well as supplemental oxygen should be implemented when performing spinal anesthesia. Fluid bolus should be carefully monitored as excess fluid may cause patients to go into congestive heart failure, pulmonary edema, or both, and also may necessitate bladder catheterization after surgery. Bladder catheterization can lead to its own set of problems, including urinary tract infections. If pharmacologic treatment of hypotension is indicated, vasopressors remain the mainstay of treatment. Combinedα- and β-adrenergic agonists may be better than pure α-agonists for treating blood pressure depression, and ephedrine is currently the drug of choice.[148,149] Cardiac output and peripheral vascular resistance are increased by ephedrine, which restores blood pressure. However, physiologic treatment of hypotension centers on restoration of preload. The most effective and simple way to achieve this is by positioning the patient in the Trendelenburg, or head down, position.[150] This position should not exceed 20 degrees because extreme Trendelenburg can lead to a decrease in cerebral perfusion and blood flow due to increases in jugular venous pressure. If the level of spinal anesthesia is not fixed, the Trendelenburg position can alter the level of spinal anesthesia and cause a high level of spinal anesthesia in patients receiving hyperbaric local anesthetic solutions.[151] This can be minimized by raising the upper part of the body with a pillow under the shoulders while keeping the lower part of the body elevated above heart level. Figure 8 shows an algorithm for the treatment of hypotension after spinal anesthesia. 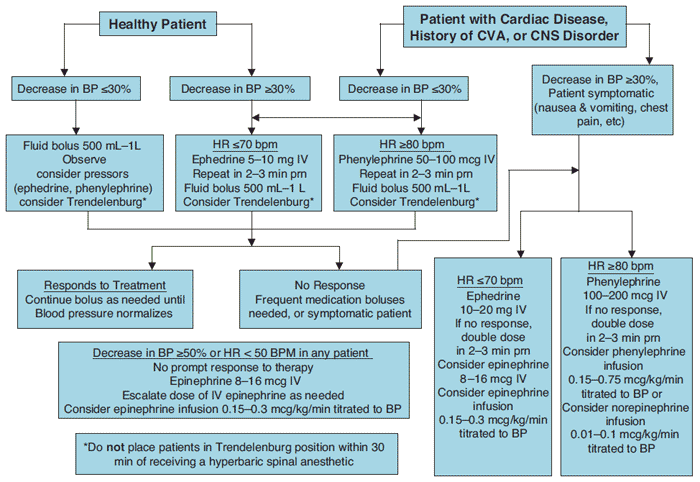
Figure 8: Treatment of hypotension after spinal anesthesia. CVA=cardiovascular accident, CNS=central nervous system, BP = blood pressure, HR = heart rate, bpm = beats per minute. The Bezold Jarisch Reflex The Bezold-Jarisch reflex (BJR) has been implicated as a cause of bradycardia, hypotension, and cardiovascular collapse after central neuraxial anesthesia, and in particular spinal anesthesia.[152,153] The BJR is a cardio-inhibitory reflex and consists of the triad of symptoms, bradycardia, hypotension, and cardiovascular collapse, seen after intravenous injection of Veratrumalkaloids in animals.[154] The BJR is usually not a dominant reflex and the association with spinal anesthesia is probably weak.[154,155] Blood pressure regulation is multimodal and complex, and while the BJR likely plays a role in this regulation, the dominant reflex in regulation of blood pressure is the baroreceptor reflex. The BJR is also not a vasovagal reflex, although BJR has been blamed for bradycardia after spinal anesthesia, especially after hemorrhage.[156] No studies have yet defined this relationship. With the dearth of data available, more research must be done before the BJR is named as the cause of bradycardia, hypotension, and circulatory collapse after spinal anesthesia. Respiratory Effects of Spinal Anesthesia In patients with normal lung physiology, spinal anesthesia has very little effect on pulmonary function.[157] Lung volumes, resting minute ventilation, dead space, arterial blood gas tensions, and shunt fraction show minimal change after spinal anesthesia. The main respiratory effect of spinal anesthesia occurs during high spinal blockade when active exhalation is affected due to paralysis of abdominal and intercostal muscles. During high spinal blockade, expiratory reserve volume, peak expiratory flow, and maximum minute ventilation are reduced. Patients with obstructive pulmonary disease that rely on accessory muscle use for adequate ventilation should be monitored carefully after spinal blockade. Patients with normal pulmonary function and a high spinal block may complain of dyspnea, but if they are able to speak clearly in a normal voice, ventilation is usually normal. The dyspnea is usually due to the inability to feel the chest wall move during respiration, and simple assurance is usually effective in allaying the patient's distress.
Arterial blood gas measurements do not change during high spinal anesthesia in patients who are spontaneously breathing room air. The main effect of high spinal anesthesia is on expiration, as the muscles of exhalation are impaired. Since a high spinal usually does not affect the cervical area, sparing of the phrenic nerve and normal diaphragmatic function occurs, and inspiration is minimally affected. Although Steinbrook and colleagues found that spinal anesthesia was not associated with significant changes in vital capacity, maximal inspiratory pressure, or resting end-tidal PCO2, an increased ventilatory responsiveness to CO2 with bupivacaine spinal anesthesia was seen.[158] Gastrointestinal Effects of Spinal Anesthesia The sympathetic innervation to the abdominal organs arises from T6 to L2. Due to sympathetic blockade and unopposed parasympathetic activity after spinal blockade, secretions increase, sphincters relax, and the bowel becomes constricted.
Nausea and vomiting occur after spinal anesthesia approximately 20% of the time, and risk factors include blocks higher than T5, hypotension, opioid administration, and a history of motion sickness.[134] Increased vagal activity after sympathetic block causes increased peristalsis of the gastrointestinal tract, which leads to nausea. Accordingly, atropine is useful for treating nausea after high spinal blockade.[149] The Use Of Spinal Anesthesia In Obstetrics In 1901, Kreis described the first spinal anesthetic for vaginal delivery.[159] Spinal anesthesia for labor and delivery has progressed greatly since that time. When contemplating induction of anesthesia in the pregnant patient, many factors play a role. The anesthesiologist must perform a complete preanesthetic evaluation, including past medical and surgical history, past reactions to anesthesia, any difficulties during the pregnancy, maternal airway and back anatomy, and fetal assessment. In addition, the anesthesiologist must obtain informed consent for both regional and general anesthesia. Before performing a spinal anesthetic on the labor floor, resuscitative equipment and emergency medication must be readily available. Although many arguments aremade against general anesthesia in the pregnant woman due to increased risk of aspiration and difficult intubation, the anesthesiologistmust be prepared to induce general anesthesia in the face of a failed or total spinal anesthetic.
Spinal anesthesia is useful in both elective and emergent cesarean sections. Non-cutting, pencil-point spinal needles are used for spinal obstetric anesthesia, which has resulted in a decreased incidence of PDPH. Most of the time, spinal obstetric anesthesia is administered as a single injection, and the rapid onset and dense neural block are of benefit. Because of the sympathetic blockade, hypotension may result, so it is prudent to monitor the blood pressure very carefully and frequently. The anesthesiologist should treat hypotension immediately with medications or fluid administration, or both. Pregnant women require less local anesthetic to achieve the same level of anesthesia as non-pregnant women. This observation is likely due to both hormonal and mechanical factors. Procaine, tetracaine, lidocaine, bupivacaine, ropivacaine, and levobupivacaine have all been used for obstetric anesthesia, but the preferred local anesthetic is bupivacaine. Dosing is generally done either with a fixed amount of local anesthetic or changing the amount according to the height and weight of the patient. If hyperbaric bupivacaine is used, 12 mg is generally given, with a decreased dose for short patients and an increased dose if the spinal is given in the sitting position. Fentanyl 10-20 mcg is usually added to enhance the quality of the block. Prior to placement of a spinal anesthetic, the pregnant patient should receive 30 mL of 0.3 M sodium citrate orally to decrease the stomach acidity, and a bolus of Ringer's lactate 15-20 mL/kg. Metoclopramide to improve gastric emptying can also be given prior to the spinal. After the spinal anesthetic is given, the patient should be in the supine position with left uterine displacement. Fetal heart rate should be monitored by Doppler or fetal scalp electrocardiogram (ECG). Blood pressure and heart rate should be monitored every minute for at least 10 min, and immediate treatment should be given for decreases in blood pressure. A T4 level block is usually required for a cesarean section due to traction on the peritoneum and uterine exteriorization. Some patients complain of dyspnea due to abdominal and intercostal motor blockade, but if the patient is able to speak clearly, assurance and possible gentle bag- and mask-assisted ventilation is usually enough to calm the patient until delivery. Once the fetus is delivered, the uterus no longer causes up ward pressure on the diaphragm, and the patient is able to breathe more easily. Factors Affecting Level Of Spinal Blockade Many factors have been suggested as possible determinants of spinal blockade level.[50] The four main categories of factors are (1) characteristics of the local anesthetic solution, (2) patient characteristics, (3) technique of spinal blockade, and (4) diffusion. Characteristics of local anesthetic solution include baricity, local anesthetic dose, local anesthetic concentration, and volume injected. Patient characteristics include age, weight, height, gender, intra-abdominal pressure, anatomy of the spinal column, spinal fluid characteristics, and patient position.[160] Techniques of spinal blockade include site of injection, speed of injection, direction of needle bevel, force of injection, and addition of vasoconstrictors (see Table 1).
Although all these factors have been postulated as affecting spinal spread of anesthetic, not many have been shown to change the distribution of blockade when all other factors that affect blockade are kept constant. Site of injection, age, position of the patient during and after injection, dosage and volume of anesthetic solution injected, baricity of local anesthetic, anatomy of the spine, direction of the needle during injection, volume of CSF, and increased intraabdominal pressure can all influence the spread of spinal blockade. Site of Injection The site of injection of local anesthetics for spinal anesthesia can determine the level of blockade. In some studies, isobaric spinal 0.5% bupivacaine produces sensory blockade that is reduced by two dermatomes per interspace when injection at L2-3, L3-4, and L4-5 interspaces are compared.[161,162]
However, no difference in block height exists when hyperbaric bupivacaine or dibucaine is injected as a spinal anesthetic in different interspaces.[163-165] Age Some studies have reported changes in block height after spinal anesthesia in the elderly patient as compared with the young patient, but other studies have reported no difference in block height.[166-169] These studies were performed with both isobaric and hyperbaric 0.5% bupivacaine.
Isobaric bupivacaine appears to increase block height, and hyperbaric bupivacaine does not appear to change block height with increasing age. If there is a correlation between increasing age and spinal anesthesia height, it is not strong enough by itself to be a reliable predictor in the clinical setting.[170,171] Just as with site of injection, it appears that baricity plays a major role in determining block height after spinal anesthesia in older populations and age is not an independent factor. Position Positioning of the patient is very important for determining level of blockade after hyperbaric and hypobaric spinal anesthesia, but not for isobaric solutions. Sitting, Trendelenburg, and prone jackknife positions can greatly change the spread of the local anesthetic due to effect of gravity.[172-174] Gravity and baricity are interrelated when position is involved in determining spinal block height.
The combination of baricity of the local anesthetic solution and patient positioning determines spinal block height level.[175] Thesitting position in combination with a hyperbaric solution can produce analgesia in the perineum. Trendelenburg positioning will also affect spread of hyperbaric and hypobaric local anesthetics due to the effect of gravity.[151,176] Prone jackknife positioning is used for rectal, perineal, and lumbar procedures with a hypobaric local anesthetic.[59,177] This prevents rostral spread of the spinal blockade after injection. Speed of Injection Speed of injection has been reported to affect spinal block height, but the data available in the literature are conflicting.178
In studies using isobaric bupivacaine, there is no difference in spinal block height with different speeds of injection.[179-181] Even though spinal block height does not change with speed of injection, a smooth, slow injection should be used when giving a spinal anesthetic. If a forceful injection is given and the syringe is not connected tightly to the spinal needle, the local anesthetic might be aerosolized and lost to the atmosphere. Volume, Concentration, & Dose of Local Anesthetic It is difficult to maintain volume, concentration, or dose of local anesthetic constant without changing any of the other variables, thus it is difficult to produce high-quality studies that investigate these variables singly. Axelsson and associates showed that volume of local anesthetic can affect spinal block height and duration when equivalent doses are used.[182]
Peng and coworkers showed that concentration of local anesthetic is directly related to dose when determining effective anesthesia.[183] However, dose of local anesthetic plays the greatest role in determining spinal block duration, as neither volume nor concentration of isobaric bupivacaine or tetracaine alter spinal block duration when the dose is held constant.[184,185] Studies have repeatedly shown that spinal block duration is longer when higher doses of local anesthetic are given.[54,61,182,186,187] When performing a spinal anesthetic, be cognizant of not only the dose of local anesthetic, but also the volume and concentration so as not to overdose or underdose the patient. The use of hyperbaric solutions minimizes the importance of dose and volume except when doses of hyperbaric bupivacaine equal to or less than 10 mg are used. In those cases, there is less cephalad spread and a shorter duration of action.[173] A dose of hyperbaric bupivacaine between 10 and 20 mg results in similar block height.[163] When using hyperbaric solutions, it is important to note that patient positioning and baricity are the most influential factors on block height, except when low doses of hyperbaric bupivacaine are used. Choice Of Local Anesthetic The choice of local anesthetic determines the duration of the spinal blockade. The shortest acting local anesthetic for spinal use is preservative-free 2-chloroprocaine.[188] Procaine is the next shortest acting local anesthetic, followed by lidocaine. The long-acting local anesthetics include bupivacaine, ropivacaine, and tetracaine. Even though chloroprocaine is not currently approved by the FDA for the specific indication of intrathecal use, results from recent clinical trials have shown preservative-free 2-chloroprocaine to be safe, short-acting, and acceptable for outpatient surgery, with some episodes of flu-like symptoms and low back pain associated with the addition of epinephrine.[88] Chronic neurologic deficits have been reported in rabbits when sodium bisulfite is injected into the lumbar subarachnoid space, but when preservative-free 2-chloroprocaine was injected, no permanent neurologic sequelae were noted.[189] Onset time is very fast, and the duration is around 60 min for surgical anesthesia. The dose ranges from 20 to 60 mg, with 40 mg as a usual dose. Procaine, commonly known as Novocain, is a short-acting ester local anesthetic. Procaine has an onset time of 3 to 5 min and a duration time of 50 to 60 min. However, there is a 14% incidence of block failure associated with procaine 10%.[190] A dose of 50 to 100 mg is suggested for perineal and lower extremity surgery. Concerns about the neurotoxicity of procaine have limited its use, but there appears to be less risk of TNS and transient radicular irritation (TRI).[191-193] Procaine can be used only for short cases.
Lidocaine, an amide local anesthetic, also provides an onset time of 3 to 5 min with a duration time of 60 to 90 min. As described previously, there is a strong association between lidocaine and TNS, which limits the usefulness of lidocaine.[65-67] For perineal surgery and saddle-block anesthesia, a dose of 25 to 50 mg is given. Lidocaine is also used for short operating room cases. Tetracaine, another ester local anesthetic, provides anesthesia within 3 to 6 min and lasts 210 to 240 min. The duration of anesthesia is much longer than with the other ester anesthetics and also much longer than with lidocaine. The suggested dose is 5 mg for perineal and lower extremity surgery. Tetracaine is used for intermediate to long lasting cases. Bupivacaine, another amide local anesthetic, has an onset time of 5 to 8 min with a duration time of 210 to 240 min, which is similar to tetracaine. The suggested dose is 8-10 mg for perineal and lower extremity surgery and 15-20mg for abdominal surgery. Bupivacaine is one of the most widely used local anesthetics for spinal anesthesia and provides adequate anesthesia and analgesia for intermediate to long duration operating room cases. Number & Frequency of Local Anesthetic Injections In the majority of cases, a single-shot injection of local anesthetic is given when a spinal anesthetic is performed. At times, a continuous spinal anesthetic is utilized with an infusion pump continuously providing medication though a spinal catheter or by giving boluses through a spinal catheter.
Procaine, commonly known as Novocain, is a short-acting ester local anesthetic. Procaine has an onset time of 3 to 5 min and a duration time of 50 to 60 min. However, there is a 14% incidence of block failure associated with procaine 10%.[190] A dose of 50 to 100 mg is suggested for perineal and lower extremity surgery. Concerns about the neurotoxicity of procaine have limited its use, but there appears to be less risk of TNS and transient radicular irritation (TRI).[191-193] Procaine can be used only for short cases. Equipment For Spinal Anesthesia In the past, most institutions had reusable trays for spinal anesthesia. These trays required preparation by anesthesiologists or anesthesia personnel to ensure that bacterial and chemical contamination would not occur. The contents of the trays did not differ from those currently available commercially, but strict attention to sterility must be maintained to ensure patient safety while using the trays.
Currently, commercially prepared, disposable spinal trays are available and are in use by most institutions. Most of these trays contain the same items: a paper towel, fenestrated drape, gauze sponges, prep solution well and sponges, medicine well, ampules of lidocaine 1% and epinephrine, standard or pencil-point needles, introducers, syringes and needles, a filter straw, povidone-iodine solution packet, needle block foam with holder, and an ampule of local anesthetic for spinal injection. These trays are portable, sterile, and easy to use. Familiarity with the contents of the spinal tray is essential to placing a spinal anesthetic quickly. Figure 9 shows the contents of standard, commercially prepared spinal anesthetic tray. 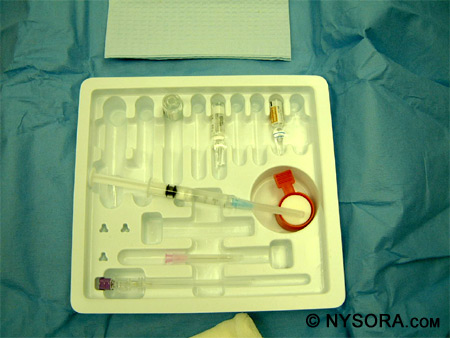
Figure 9: The contents of standard, commercially prepared spinal anesthesia tray. Resuscitation equipment must be available whenever a spinal anesthetic is performed. This includes medication for sedation and induction of general anesthesia (propofol, fentanyl, midazolam, succinylcholine), medication for support of cardiac function (ephedrine, epinephrine, atropine), an oropharyngeal airway, a laryngoscope with blade, an endotracheal tube with stylet and cuff syringe, tape for securing the endotracheal tube, a tongue depressor, a Yankauer suction tube, an oxygen source, and an Ambu bag and facemask. The patient must be monitored during the placement of the spinal anesthetic with a pulse oximeter, blood pressure cuff, and ECG. All of these precautions are necessary to provide the safest environment for performing a spinal anesthetic. Needles Needles of different diameters and shapes have been developed for spinal anesthesia. The ones currently used have a close-fitting, removable stylet, which prevents skin and adipose tissue from plugging the needle and possibly entering the subarachnoid space. Figure 10 shows the different types of needles used along with the type of point at the end of the needle. 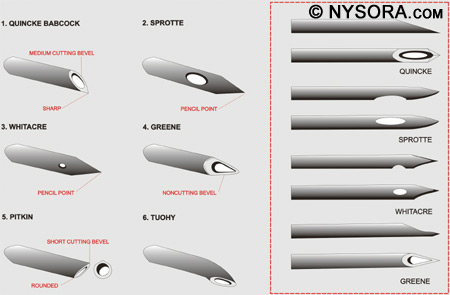
Figure 10: The different types of needles used for spinal anesthesia along with the type of point at the end of each type of needle. The pencil-point needles (Sprotte and Whitacre) have a rounded, noncutting bevel with a solid tip. The opening is located on the side of the needle 2-4 mm proximal to the tip of the needle. The needles with cutting bevels include the Quincke and Pitkin needles. The Quincke needle has a sharp point with a medium-length cutting needle, and the Pitkin has a sharp point and short bevel with cutting edges. Finally, the Greene spinal needle has a rounded point and rounded noncutting bevel. If a continuous spinal catheter is to be placed, a Tuohy needle can be used to find the subarachnoid space before placement of the catheter. Pencil-point needles provide a better tactile sensation of the layers of ligament encountered but require more force to insert than bevel-tip needles. The bevel of the needle should be directed longitudinally to decrease the incidence of PDPH.[195]
Larger gauge needles and needles with rounded, noncutting bevels also decrease the incidence of PDPH, but are more easily deflected than smaller gauge needles. Introducers have been designed to assist with the placement of spinal needles into the subarachnoid space due to the difficulty in directing needles of small bore through the tissues. Introducers also serve to prevent contamination of the CSF with small pieces of epidermis, which could lead to the formation of dermoid spinal cord tumors. The introducer is placed into the interspinous ligament in the intended direction of the spinal needle, and the spinal needle is then placed through the introducer. Position Of The Patient Proper positioning of the patient for spinal anesthesia is essential for a fast, successful block. Many factors come into play for positioning of the patient. Before beginning the procedure, both the patient and anesthesiologist should be comfortable. This includes a proper level of the operating room table, adequate blankets or covers for the patient, a functioning intravenous line, standard American Society of Anesthesiologists (ASA) monitors, administration of 100% oxygen, and sedation for the patient.
A trained assistant should be available to help optimize patient position. The patient should receive some sedation, but not too much, in order to be comfortable during the procedure. The patient should be able to cooperate before, during, and after administration of the spinal anesthetic. There are three main positions for administering a spinal anesthetic: the lateral decubitus, sitting, and prone position. Lateral Decubitus Position The most commonly used position for placing a spinal anesthetic is the lateral decubitus position. Ideal positioning consists of having the back of the patient parallel to the edge of the bed closest to the anesthesiologist, knees flexed to the abdomen, and neck flexed. Figure 11 shows a patient in the lateral decubitus position.
It is essential to have an assistant to help hold and encourage the patient to stay in this position. Depending on the operative site and operative position, a hypo-, iso-, or hyperbaric solution of local anesthetic can be injected. 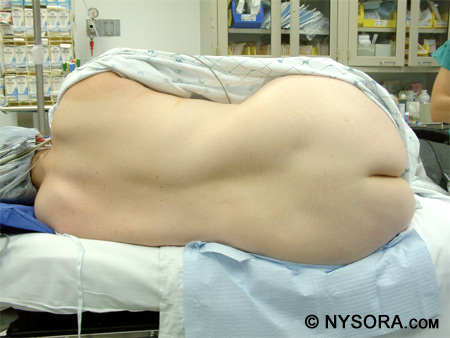
Figure 11: A patient in the lateral decubitus position. Sitting Position & "Saddle Block" Strictly speaking, the sitting position is best utilized for low lumbar or sacral anesthesia and in instances when the patient is obese and there is difficulty in finding the midline in the lateral position. In practice, however, many anesthesiologists prefer the sitting position in all patient who can be positioned for the ease of identification of the landmarks. Using a stool for a footrest and a pillow for the patient to hold can be valuable in this position. The patient should have the neck flexed and push out the lower back to open up the lumbar vertebral space. Figure 12 depicts a patient in the sitting position and the L4-5 interspace is marked. 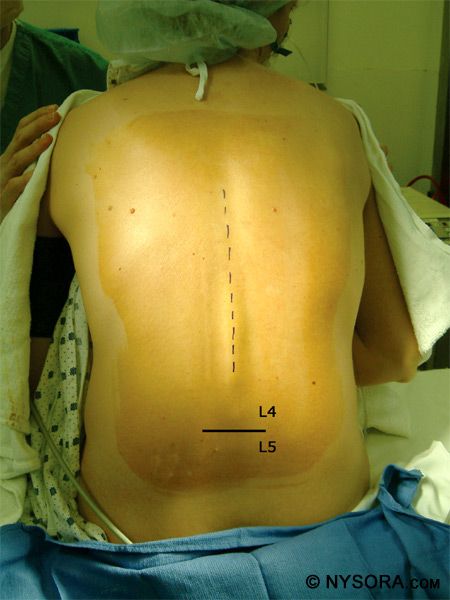
Figure 12: A patient in the sitting position with the L4/L5 interspace marked.
When performing a saddle block, the patient should remain in the sitting position for at least 5 min after a hyperbaric spinal anesthetic is placed to allow the spinal to settle into that region. If a higher level of blockade is necessary, the patient should be placed supine immediately after spinal placement and the table adjusted accordingly. Prone Position The prone position is utilized for spinal anesthesia if the patient needs to be in this position for the surgery, such as for rectal, perineal, or lumbar procedures. A hypobaric or isobaric solution of local anesthetic is preferred in the prone jackknife position for these procedures.
This allows the anesthetic to spread in the caudal direction and avoid rostral spread and the risk of high spinal anesthesia. Care should to be taken to keep the patient in the same position for at least 15 min after injection prior to moving so that the local anesthetic solution will not migrate to a level that it was not intended to be. Another, less elegant solution is to inject a hyperbaric solution of local anesthetic with the patient in the sitting position and await until the spinal anesthesia “sets-in,” which is typically 15-20 min after injection. The patient is then positioned in the prone position with vigilant monitoring, including frequent verbal communication with the patient. |
| 06/17/2016 (+ 2016 Dates) | |
| 06/11/2024(+ 2016 Dates) | |
| 09/23/2016 | |
| 9/30/2016 | |
| 11/03/2024 |
![[advertisement] gehealthcare](files/banners/banner1_250x600/GEtouch(250X600).gif)






















Post your comment